CHAPTER 13
Opioids
JEFFREY FUDIN, BS, PharmD, FCCP
RUTH J. PERKINS, BS, MA, PharmD, BCPS
ARTHUR G. LIPMAN, BS, PharmD, FASHP
OVERVIEW
Opioids are among the oldest documented medications used by humans. All opioids are derivatives of pharmacologically active alkaloids from the milky exudate of the opium poppy. These drugs act by binding at opioid receptors found in the CNS, the colon, and to a lesser extent, the periphery. Mu and kappa opioid receptors have clinical utility and delta opioid receptors offer promise, but no delta agonist has been found acceptable for human use to date. However, new work with biased legends offers promise of a possible future delta opioid agonist analgesic. Most clinically useful opioids are mu agonists that also have varying agonist activity at kappa receptors. The mu-1 aspect of the receptor is responsible primarily for analgesia and the mu-2 for other, largely adverse, opioid effects. Numerous subtypes of the mu-1 receptors have been isolated and cloned, clearly indicating genetic polymorphism. Recent work on opioid agonist G protein coupled receptors, and specifically beta arrestin, has elucidated our understanding of biased ligands that helps explain the mechanisms by which opioids cause some adverse effects. This offers promise of new agents which offer full analgesia with fewer adverse effects.1-5
The majority of opioid agents are indicated and FDA-approved for management of acute and chronic pain. However, some have indications other than pain (e.g., naloxone for opioid overdose reversal, naltrexone for abuse mitigation, naloxegol for opioid-induced constipation (OIC), buprenorphine for maintenance therapy in patients with a history of substance abuse, dextromethorphan and codeine for cough, diphenoxylate, codeine, and loperamide for diarrhea). In order to better understand and differentiate the therapeutic differences among natural, semisynthetic, and synthetic opioids, one must first appreciate the physiology and pharmacology associated with the class. This chapter will focus on those medications used specifically for analgesia but will include buprenorphine, as it too is indicated for pain.
These medications fall generally into four chemical classes: phenanthrenes, benzomorphans, phenylpiperidines, and diphenylheptanes. A fifth hybrid class of synthetics has some chemical similarities to several of these four groups. These dimethylamino compounds include tramadol and tapentadol and are seen in Figure 13-1. The chemical class of an opioid has little effect on its clinical utility. Note that Figure 13-1 lists cross-sensitivities as probable, possible, or low risk for each class from left to right. Although a true allergic reaction to any opioid is rare, pruritis is quite common. Pruritic reactions are a result of histamine release from mast cells. Such a reaction to one chemical class subjects a patient to histamine reactions to opioids within the same class. The fentanyl family has minimal histamine reactivity compared to all other opioids.6
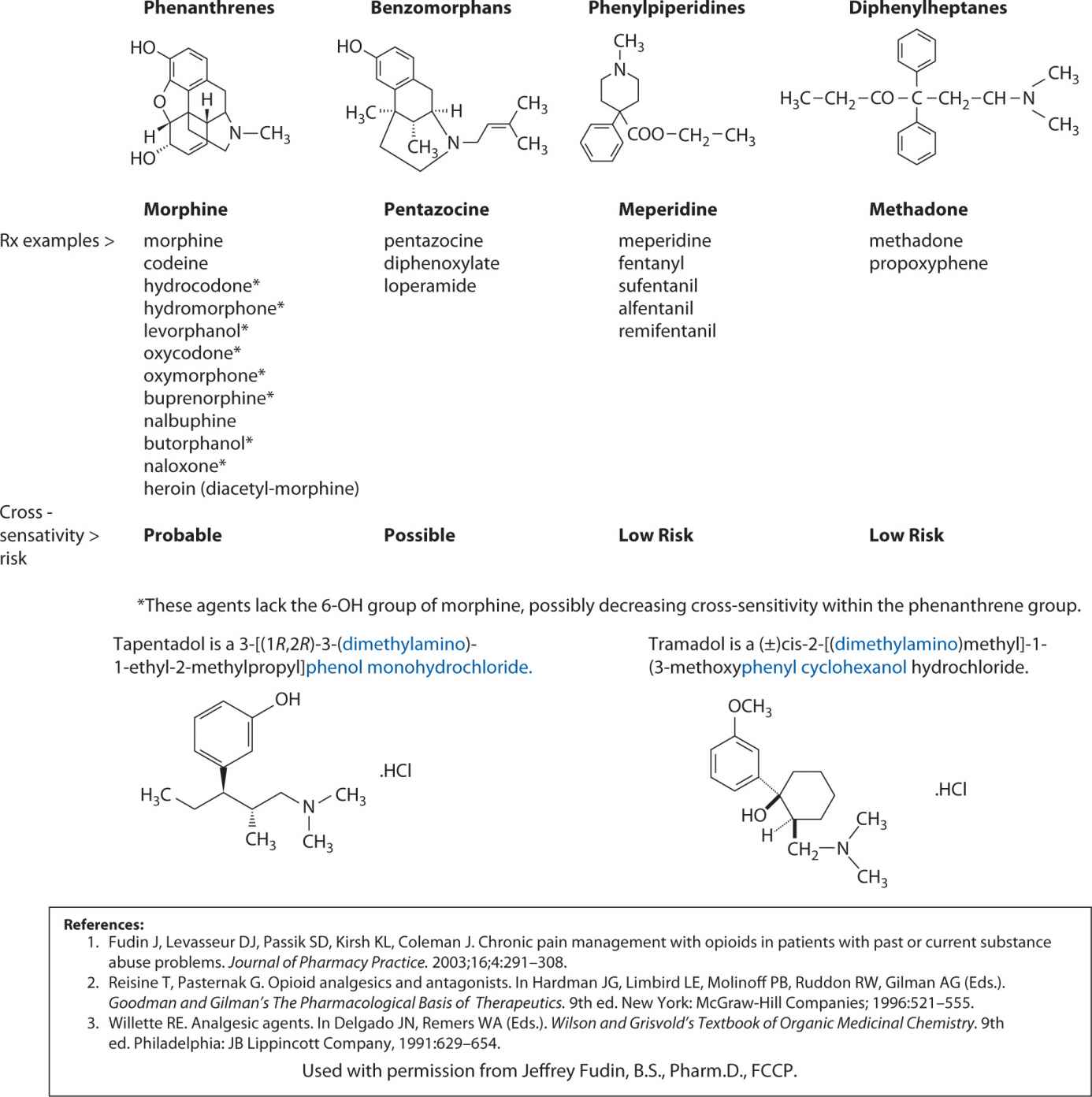
FIGURE 13-1. Chemical Classes of Opioids
Synthetic and semisynthetic opioids exhibit the same pharmacological properties as naturally occurring opium alkaloids and derivatives. Synthetic opioids do not contain the traditional phenanthrene nucleus found in the alkaloids isolated from opium. Available opioids all have similar activity, but vary considerably in potency, solubility, dosage form availability, and pharmacokinetics. Potency and solubility as outlined in Tables 13-1 through 13-4 do not generally impact therapeutic utility. However, when a small-volume opioid solution is desirable for parenteral administration due to volume restriction required because of comorbidity or for a continuous subcutaneous infusion, solubility becomes important.
For acute pain in an otherwise opioid-naive patient, it is generally best to initiate therapy with the lowest recommended dose proportionate to the intensity of the pain to assess tolerability and efficacy. The most common side effects are nausea and vomiting, constipation, sedation, urinary retention, and respiratory depression. For the chronic pain patient, sedation and respiratory depression are generally of less concern because some degree of tolerance develops to these relatively quickly. Conversely, constipation and urinary retention could remain an ongoing problem. For this reason, many acute and chronic pain patients often need stimulating laxatives during a course of opioid therapy. Depending on the dose, laxatives can sometimes be avoided if the patient is instructed to drink plenty of water and increase dietary fiber.
Nearly half of chronic nonmalignant pain patients do not have adequate bowel evacuation even with fiber, fluids, and stimulating laxatives.7 For those patients, methylnaltrexone might be considered. It is currently approved for opioid-induced constipation as a subcutaneous injection in advanced disease patients and clinical trials are now ongoing with an oral form in CNMP patients.8,9 Recent approval of naloxegol, a pegylated form of naloxone chemically similar to nor-oxymorphone was recently FDA approved for OIC in chronic non-cancer pain. It will be available at 12 mg and 25 mg tablets to be taken once daily. These drugs represent a new class known as peripherally active mu opioid receptor antagonists (PAMORAs).10
Opioids are available in a variety of dosage forms, including oral, transmucosal (buccal tablets, effervescent, lozenge), transdermal, intranasal, rectal, and parenteral (intravenous, subcutaneous, intramuscular, epidural, intrathecal). Moreover, because of genetic polymorphism and because some chronic pain patients (the minority) develop pharmacologic tolerance to opioid analgesia, clinical dosages vary widely.
THERAPEUTIC AND TOXIC CONCENTRATIONS
It is difficult to assign a “therapeutic concentration” or even range to most opioids because the dosage and corresponding blood levels required for adequate analgesia is variable and patient specific. Reported therapeutic and toxic serum levels for opioids overlap. For example, a starting morphine dose of 15 mg orally every 4 hours in an opioid-naive patient could cause significant lethargy. If the same dose were used to replace another patient’s oxycodone sustained-release 160 mg PO every 12 hours, we would likely see withdrawal symptoms with little or no pain relief.
An important consideration in postmortem analysis is that decedents are often incorrectly assigned “narcotic overdose” as “cause of death” upon pathologist review. This conclusion could potentially be problematic for two reasons. The first is that postmortem blood analysis often yields higher numbers than antemortem samples due to redistribution of tissue.11,12 For this reason, it is generally less accurate to obtain cavity blood from the heart or the pulmonary vessels to specifically match dose to concentration. More accurate specimens are achievable from the subclavian or femoral veins. Secondly, if the patient’s opioid dose had been adjusted upward over time because of physical tolerance, what might otherwise appear as a lethal blood level in one patient will not necessarily correlate to death in another.
Nevertheless, important information can be gleaned from monitoring serum opioid levels clinically. These analyses have been used to monitor compliance, assess the possible effect of serum levels of one or more medications on the opioid, to compare pharmacokinetic parameters of single opioid formulation to alternative dosage forms, and to compare single opioid formulations to extemporaneously formulated products as single or combination formulations. The latter has become especially important with the newly instituted risk evaluation and mitigation strategies (REMS) imposed by the Food and Drug Administration (FDA).
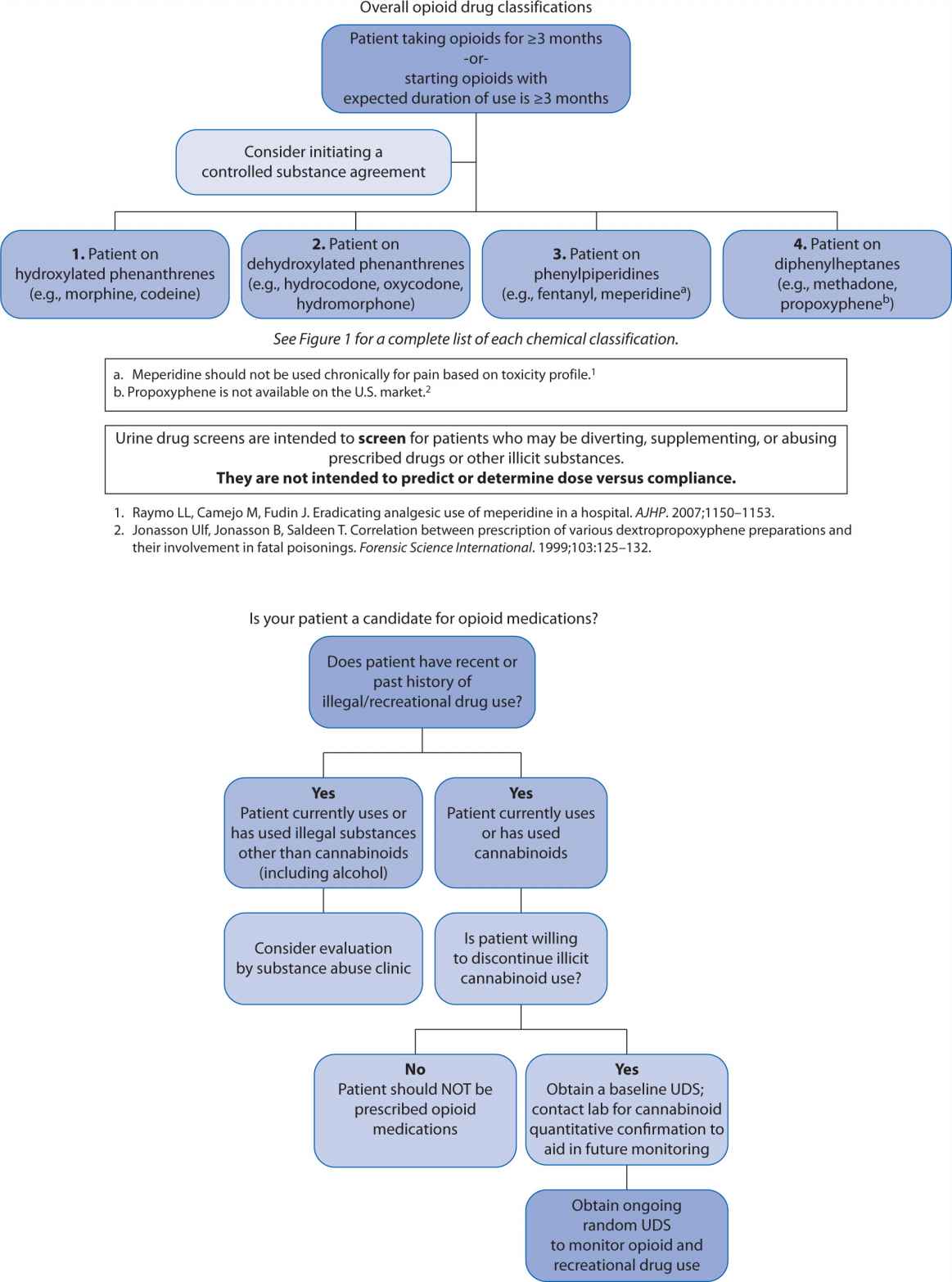
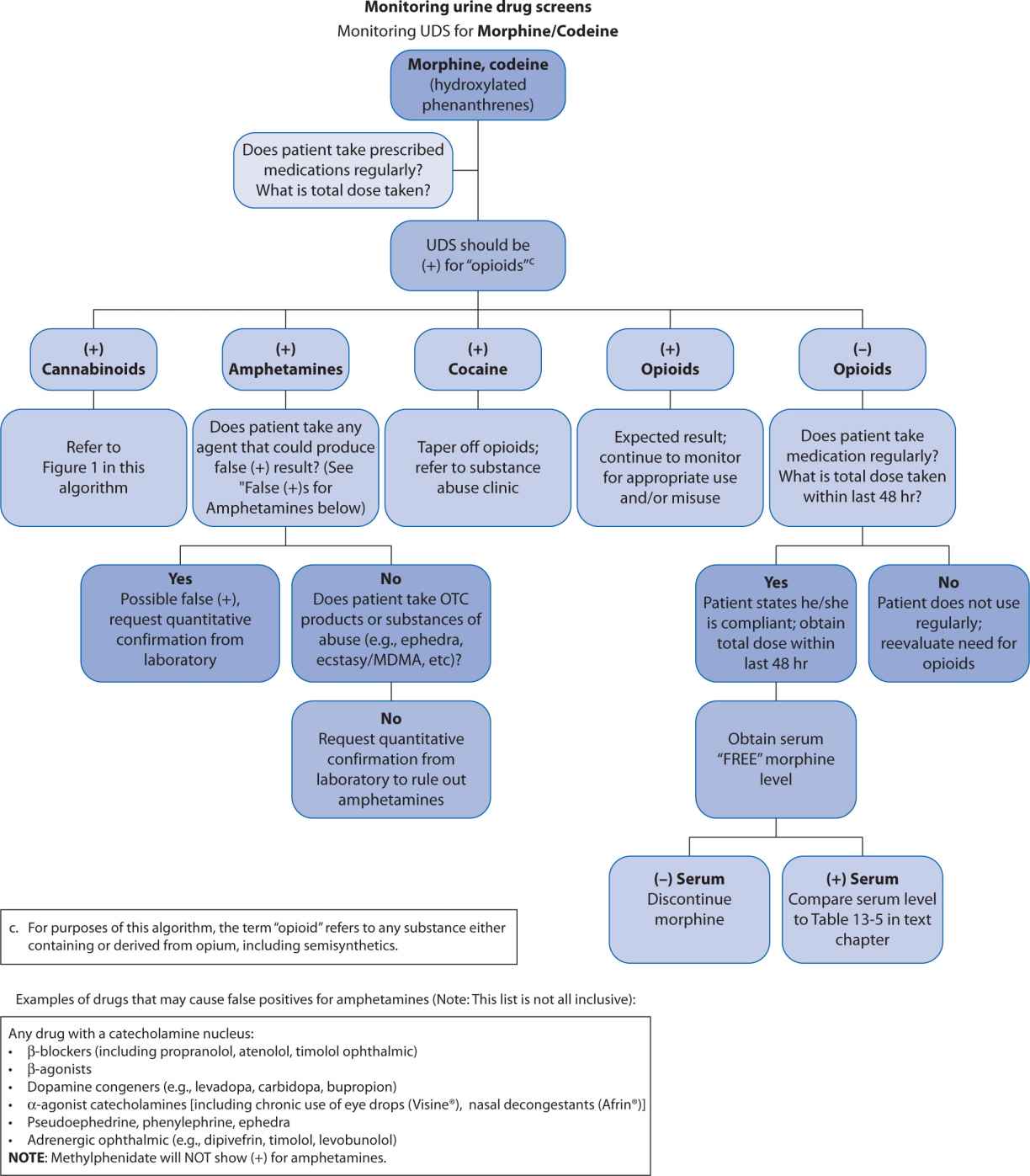
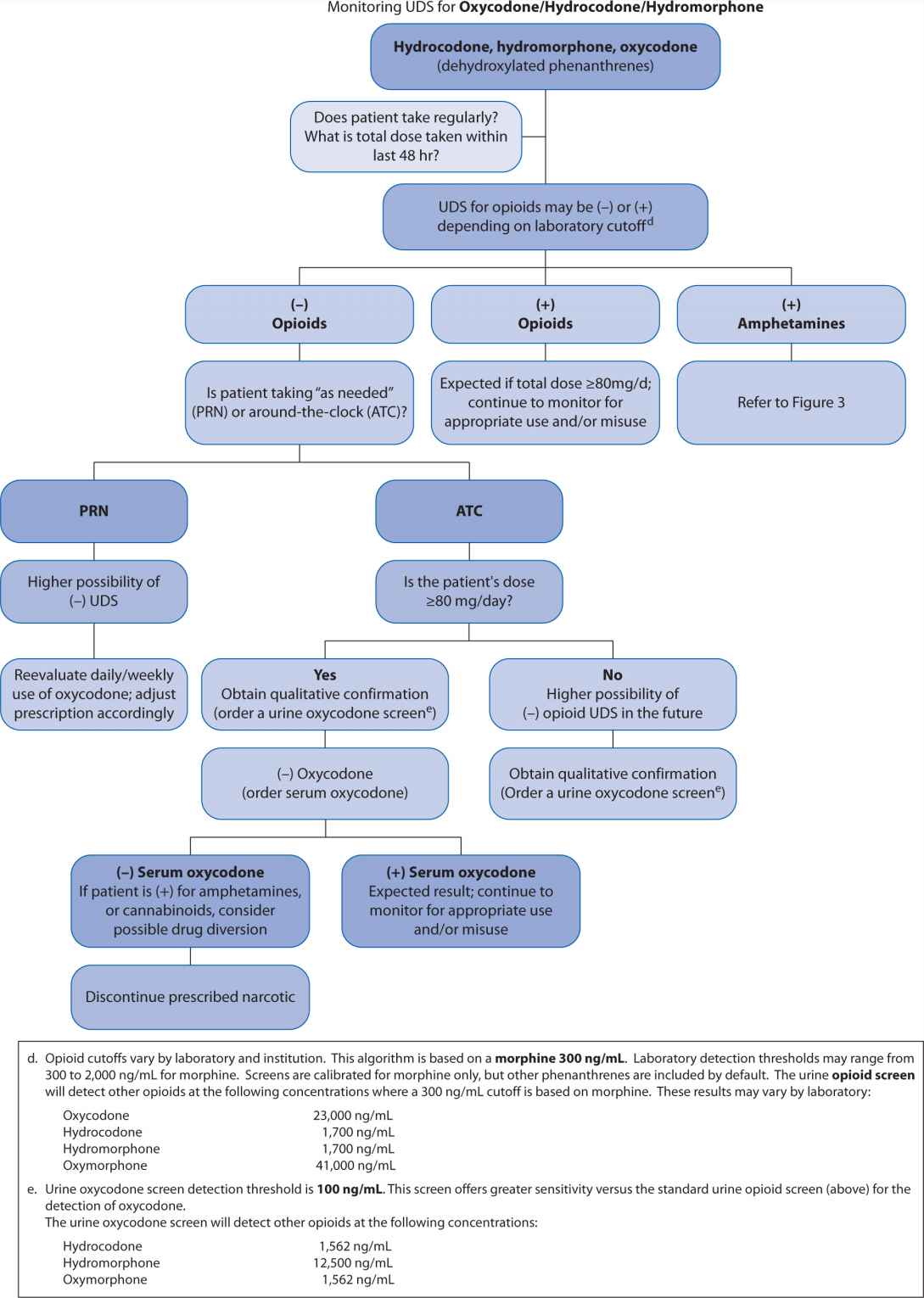
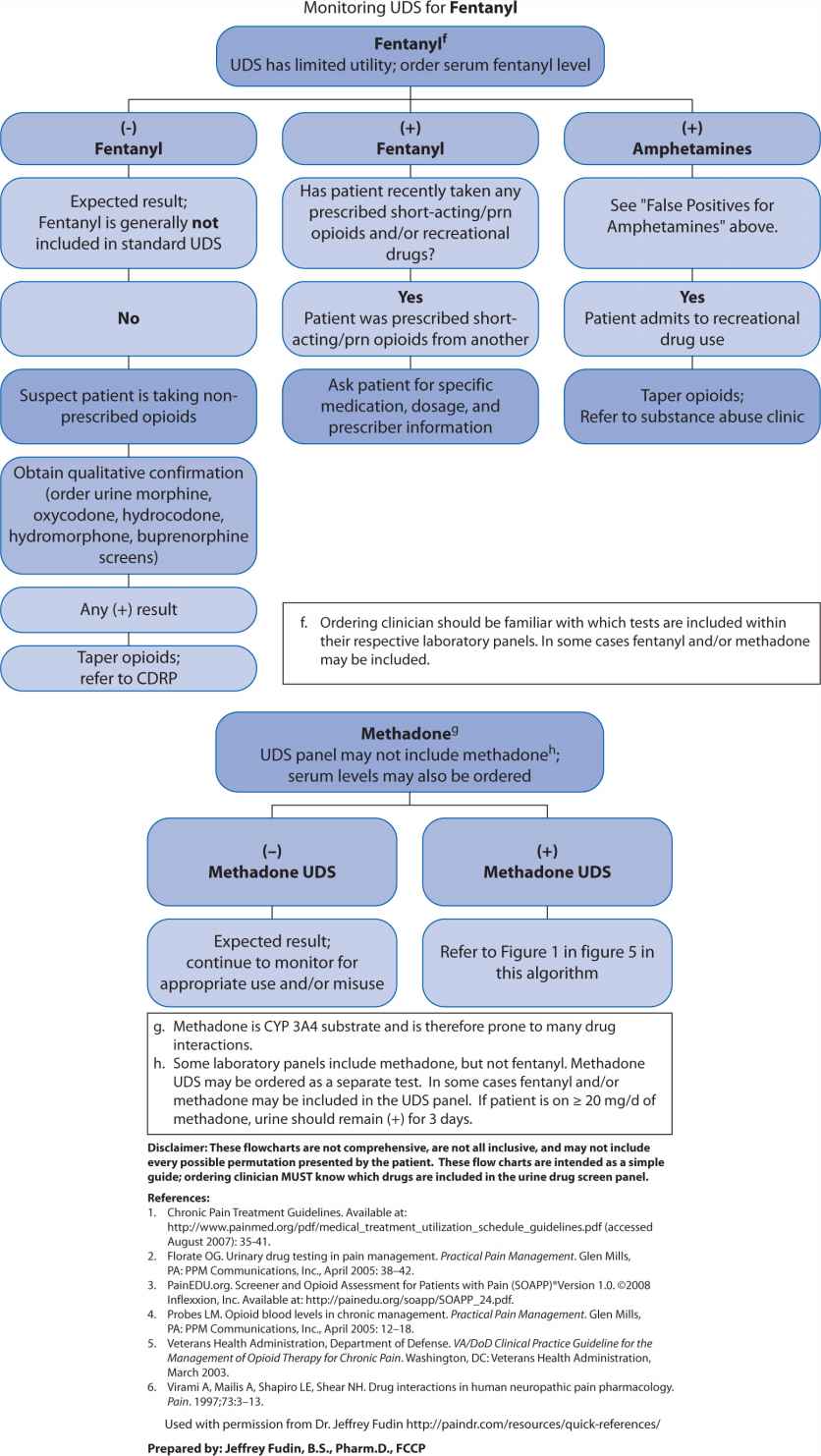
A far less accurate, but popular strategy for monitoring opioid compliance is urine drug screen (UDS) analysis. These screens present an important fallacy in that they were initially developed to assess subjects for substances of abuse. Although it is important to ascertain whether a patient is abusing recreational drugs while concomitantly receiving prescribed opioids, UDSs are almost always enzyme-type screens where false positives and false negatives are ubiquitous. Because of potential for inaccurately assigning blame for noncompliance, the clinician needs to have a clear understanding of how to interpret these tests and the potential limitations. A suggested algorithm is outlined in Figure 13-2. Some authors, notably Gourlay and Heit,13 advocate using the UDS as a “universal precaution,” but the UDS is not always acceptable, may have legal implications, and may be inappropriate for some patients.14
FIGURE 13-2. Urine Drug Screen (UDS) Algorithm
AVAILABLE ASSAYS FOR DRUG-LEVEL MONITORING
Gas (or liquid) chromatography-mass spectrometry (GCMS, LCMS) are popular tests that may be employed to accurately measure specific opioids or substances of abuse. When using such tests, it is important to recognize the importance, practicality, and implications of the measurable metabolites as well. (See Table 13-5.) Assuming regular usage for example, if a patient is receiving codeine, we would expect to find measurable morphine metabolite; if a patient is receiving oxycodone we would expect to find measurable oxymorphone metabolite; if a patient is receiving fentanyl we would expect to find measurable norfentanyl metabolite; but if a patient is receiving morphine we should not expect the presence of, for example, meperidine, fentanyl, or methadone. (see Table 13-5, Figure 13-1)
| TABLE 13-1 | Pharmacodynamic and Pharmacokinetic Properties of Commonly Prescribed Opioids |
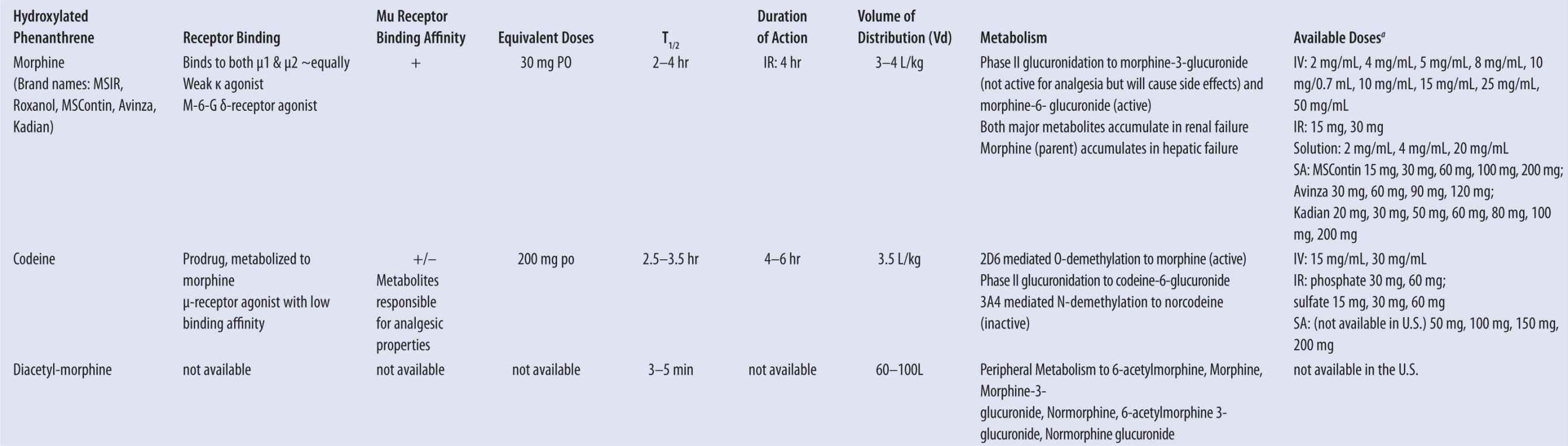
Source: Jeffrey Fudin, B.S., Pharm.D., FCCP. Ackowledgment: Jennifer L. Roy, Pharm.D. Candidate 2010.
Data from MSIR [package insert]. Perdue Pharma, Stamford, CT; Oct 2004. | MS Contin [package insert]. Perdue Pharma, Stamford, CT; March 2009. | Buprenex [package insert]. Richmond, VA: Reckitt Benckiser Pharmaceuticals 20Inc; April 2005. | Suboxone/Subutex [package insert]. Richmond, VA: Reckitt Benckiser Pharmaceuticals Inc; 2006. | Lacy CF, ed. Drug Information Handbook. 17th ed. Hudson, OH: Lexi-Comp; 2008. | Methadose [package insert]. Hazelwood, Mo: Mallinckrodt Inc; 2009. | Duragesic [package inset]. Raritan, NJ: PriCara; July 2009. | OxyContin [package insert]. Stamford, CT: Purdue Pharma; April 2010. | Molina DK, Hargrove VM. What is the lethal dose of hydrocodone? Am J Forensic Med Pathol. 2010;31(3). | Koska AJ et al. Pharmacokinetics of high-dose meperidine in surgical patients. Anesth Analg. 1981;60(1). | Nucynta [package insert]. Raritan, NJ: Pricara; March 2010. | Rook E et al. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: Review of the literature. Curr Clin Pharmacol. 2006;1:109–118. | Mayyas F et al. A systematic review of oxymorphone in the management of chronic pain. J Pain Symptom Manage. 2010;39(2):296–308.
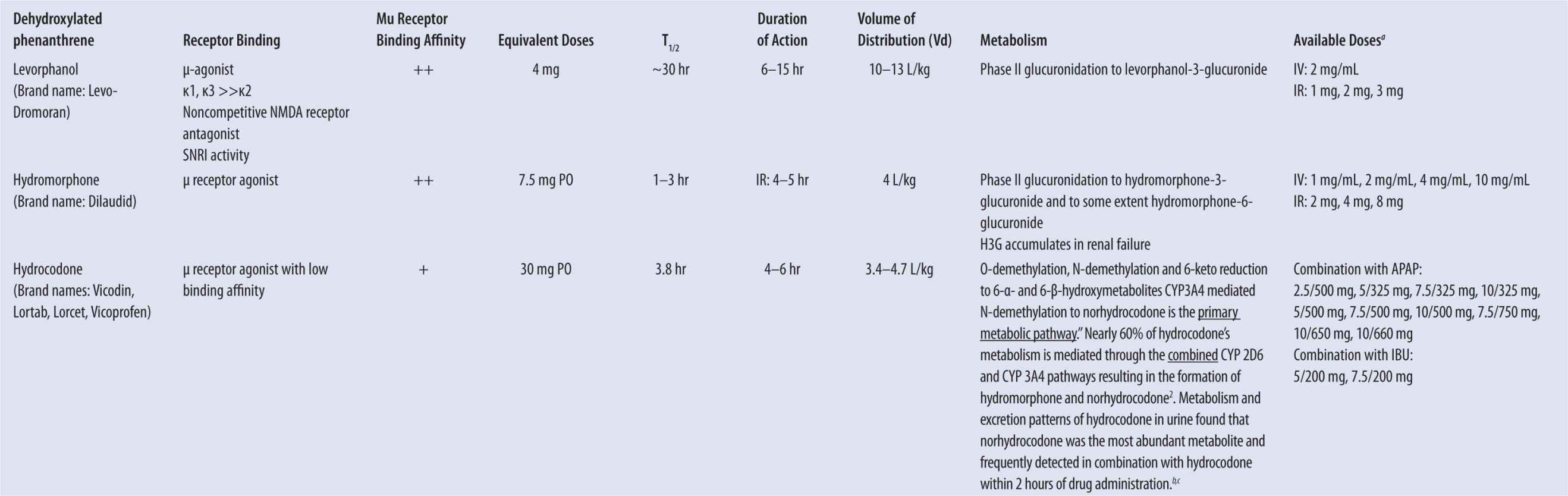
aMost common dosage forms listed; not all inclusive.
bCone EJ, et al. Prescription opioids. II. Metabolism and excretion patterns of hydrocodone in urine following controlled single-dose administration. J Anal Toxicol. 2013;37(8):486–494.
cValtier S and Bebarta VS (2012) Excretion profile of hydrocodone, hydromorphone and norhydrocodone in urine following single dose administration of hydrocodone to healthy volunteers. J Anal Toxicol. 36:507–514.
Gold MS, Redmond DE Jr, Kleber HD. Clonidine blocks acute opioid-withdrawal symptoms. Lancet. 1978;312(8090):599–602.
| TABLE 13-2 | Opioid Analgesic Comparison Table |
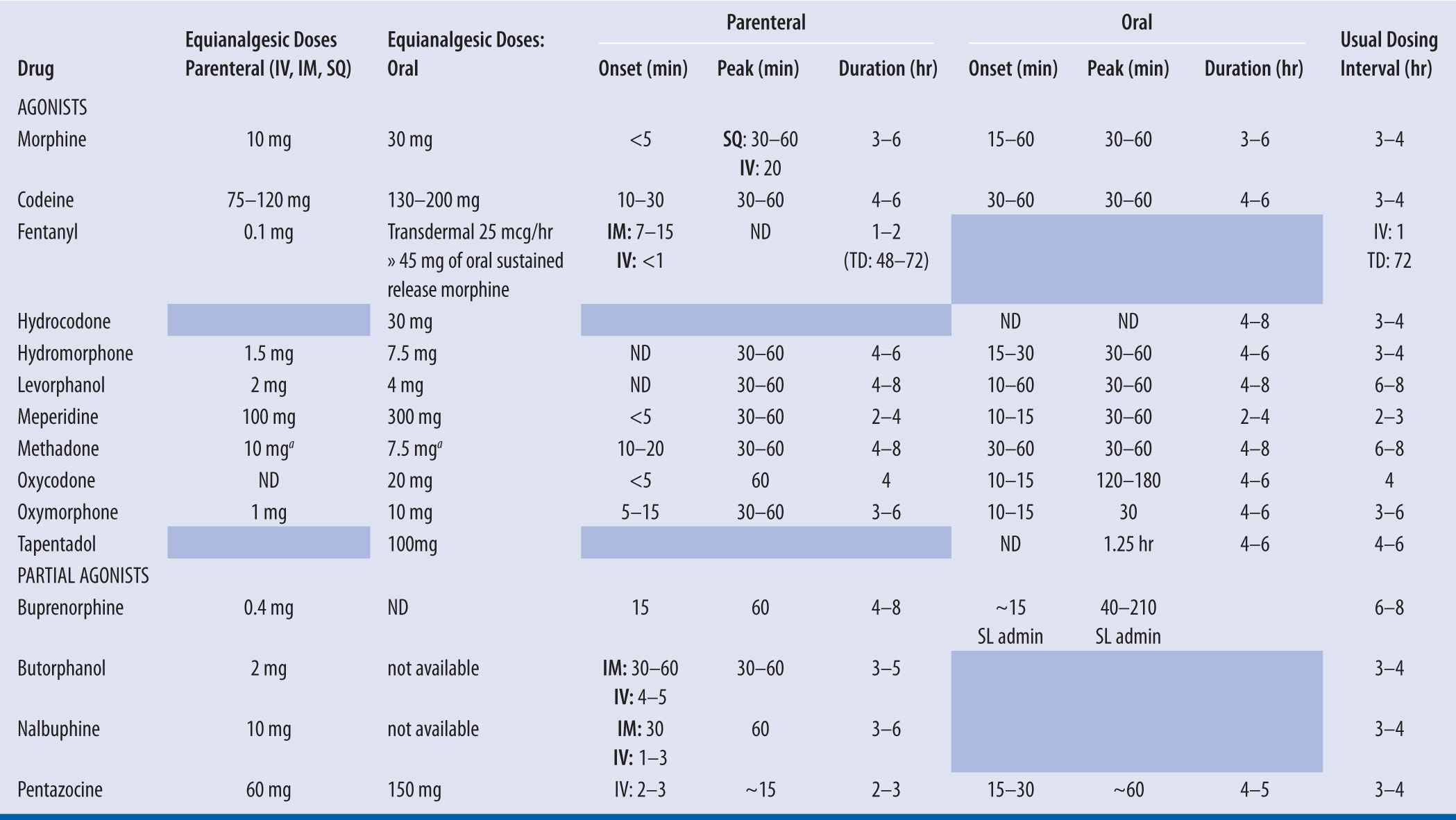
++The ratio of PO morphine: PO methadone is dependent on the dose of morphine prior to switching to methadone. With low doses of morphine (<90 mg/day), the ratio is approximately 4:1 (morphine: methadone). With higher doses of morphine (i.e., >300 mg/day), the ratio of PO morphine: PO methadone approaches 12:1. In between, a ratio of 8:1 has been studied. (Davis, 2001).
The Fudin Factor equation eliminates significant peaks and troughs associated with previously accepted schematics and employs the most conservative dosing approach at morphine equivalents up to 300 mg per day.
Fudin J, Marcoux MD, Fudin JA. Mathematical model For methadone conversion examined. Pract Pain Manag. 2012;12(8):46–51.
ND – not determined.
The opioid analgesic comparison chart is meant to act as a guideline when switching patients from one opioid to another. It is important to recognize that response to opioids varies widely among individuals. Therefore, all doses should be titrated to effect for individual patients (Foley 1985; Pereira 2000).
Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–687.
Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73–83.
Foley K. The treatment of cancer pain. NEJM. 1985;313:84–95.
| TABLE 13-3 | Dosage Recommendations for Opioids |
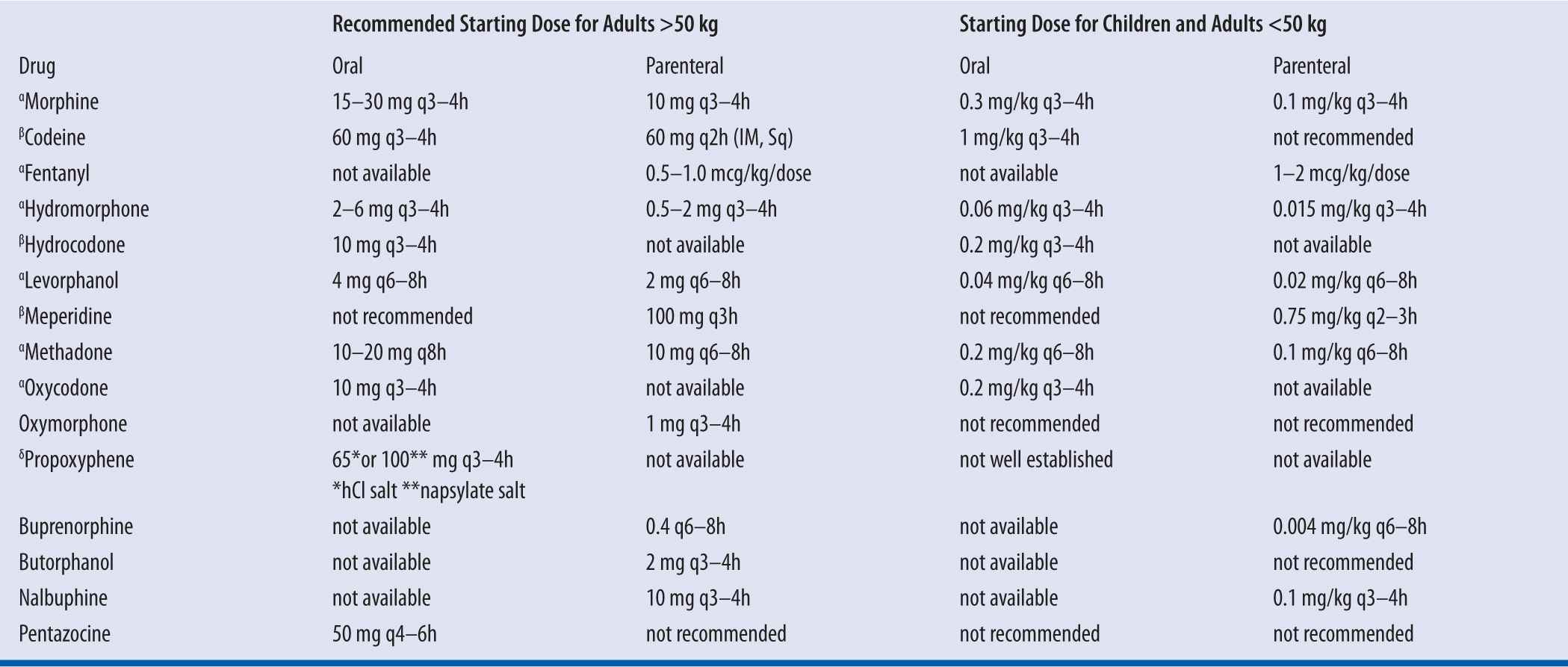
αFor treatment of moderate to severe pain; βFor treatment of mild-to-moderate pain; δFor treatment of mild pain, may be no more effective than ASA
Constipation
Because tolerance does not develop to narcotic-induced constipation, preventative measures should be initiated on day 1 of narcotic therapy. A stimulant laxative (e.g., Milk of Magnesia, senna) is necessary because opioids decrease propulsive contractions of the small intestine and increase tone of the large intestine, which slows transit time. Stool softeners as monotherapy for opioid-induced constipation are ineffective.
Meperidine should be reserved for brief courses in otherwise healthy patients who have demonstrated an unusual reaction or allergic response during treatment with other opioids (e.g., morphine, hydromorphone).
Converting patients from one opioid to another
Using conversion table, convert to morphine equivalents, then to opioid of choice. It is recommended to decrease the dose of the new opioid in half (after calculations) due to incomplete cross-reactivity between opioids in terms of analgesia and respiratory depression. Titrate up as necessary.
| TABLE 13-4 | Orally Administered Fentanyl Products |
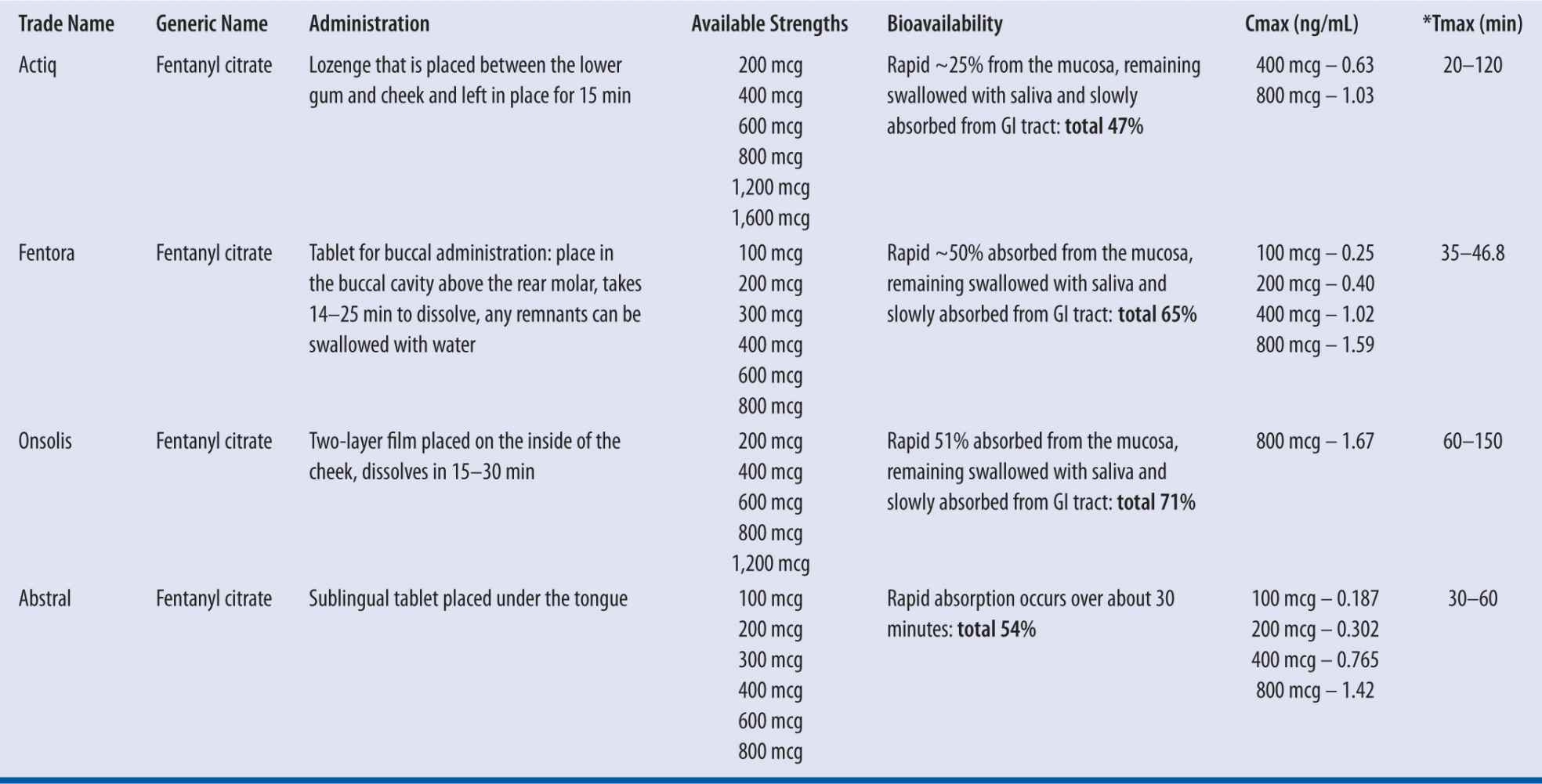
*Dose dependent; mucositis does not appear to affect peak plasma concentrations.
These products must not be used in opioid nontolerant patients due to the risk of life-threatening hypoventilation. They are not approved for the treatment of acute pain (e.g., postoperative, dental, migraine). In addition, switching between these fentanyl products must not occur at a 1:1 ratio due to differences in drug delivery and absorption profiles. Two of these products (Abstral and Onsolis) require REMS monitoring by the FDA and prescriber enrollment.
Prepared by: Ruth Perkins, B.S., M.A., Pharm.D., BCPS. Rev 04/2011
Because of the enormous variability among opioids with regard to pharmacokinetics, therapeutics, and pharmacodynamics, this chapter provides a number of comprehensive tables. It is important to understand that any conversion tables within or outside the confines of this text reflect population averages, and—due to genetic polymorphism—individual patient conversions may vary considerably. Tables 13-2 and 13-3 can be used as a starting point, but titration to response is indicated.
Additionally, due to incomplete cross-tolerance among opioids (which is only an issue in the first 7–14 days of regularly scheduled dosing), clinicians should reduce the calculated equianalgesic conversion starting dose by 20 to 25 percent ; this practice will serve to reduce risk of inadvertent overdose, especially if the patient is more sensitive to the new opioid than the one it is replacing.
Table 13-5 illustrates various commonly prescribed opioids and their corresponding expected serum levels. Note that the half-life of most commonly prescribed immediate-release products is similar. Examples include morphine, codeine, hydrocodone, hydromorphone, oxycodone, oxymorphone, and others. Figure 13-1 provides a chemical categorization of these opioids. Those with high volumes of distribution and longer half-lives include methadone and levorphanol. Methadone has a unique pharmacokinetic profile, with broad patient metabolism variations and multiple metabolites. And unlike other opioids, the majority of methadone metabolism is mediated by CYP450 3A4 isoenzymes. Therefore, it presents an important therapeutic and potentially toxic conundrum, particularly if a potent 3A4 inducer or inhibitor is introduced or abruptly discontinued. Moreover, methadone has two enantiomers (R– and S–). The “S” isomer depends on metabolism by CYP 2B6 isoenzymes and the accumulation of the “S” enantiomer is known to elevate risk of Torsade de pointes. Therefore, patients that are poor CYP 2B6 metabolizers are at higher risk of ventricular tachycardia due to accumulation of the “S” enantiomer.15 When one considers that HIV pain is commonly caused by the AIDS virus itself and/or the antiretroviral agents used to treat it, methadone analgesia, maintenance, or both is relatively common. Because almost all of the antiretrovirals significantly affect several of the cytochrome P450 isoenzymes, use of methadone requires a clear understanding of these pharmacokinetic interactions.
| TABLE 13-5 | Opioid Pharmacokinetics and Expected Metabolites |
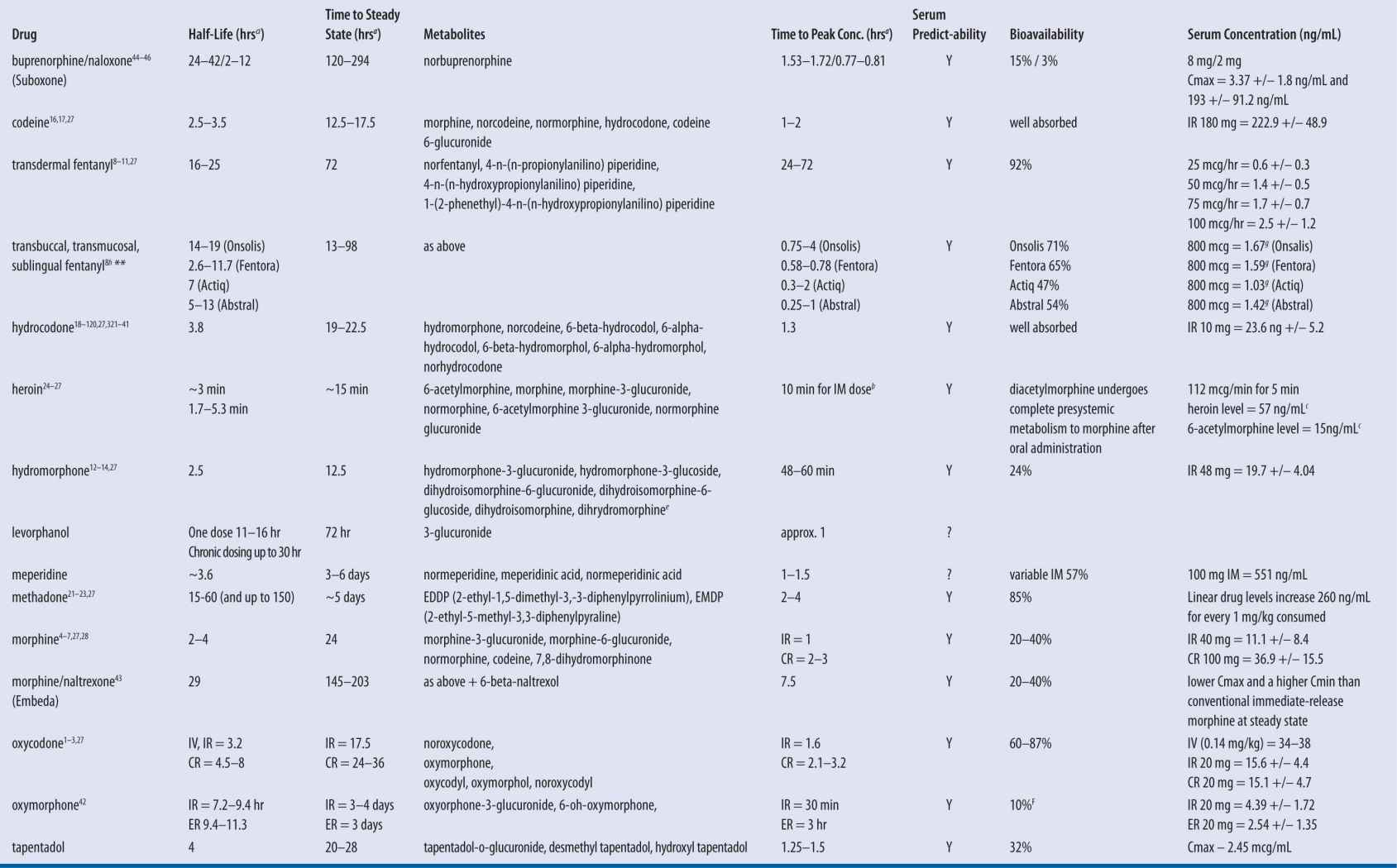
IR, immediate release products; CR, continuous release products; SS, steady state
aHours, unless otherwise indicated.
bCan detect heroin and 6-acetyl morphine within 10–15 minutes of parenteral administration.
cAdministered IV in a single patient over 180 minutes.
dCumulative amount of fentanyl release from patch dose in 24 hours.
SPECIAL CONSIDERATIONS FOR TRANSDERMAL FENTANYL
Pharmacokinetic interpatient and intrapatient variability can be significant. The elimination half-life after fentanyl transdermal patch removal is approximately 17 hours, ranging from 13 to 22 hours.16 The extended half-life is due to slow release of drug from the skin depot, which is common with all transdermal delivery systems.
Another important consideration is that elimination half-life in the elderly may be prolonged.16 This factor is especially noteworthy when switching a patient from fentanyl to an alternative opioid based solely on mathematical calculations because of the risk of overdosing the patient based on an incorrect presumption that the fentanyl is being absorbed. Homework case 2 illustrates this point when converting from fentanyl. The transition from fentanyl to the new opioid should be slow with use of immediate-release products for 2–4 days, until the lowest possible pain level is maintained. After the transition, a sustained-release formulation may be introduced. Following patch removal, the analgesic effects of fentanyl may continue for 12 to 24 hours.17
Absorbed transdermal fentanyl is proportional to the surface area of the patch. Fentanyl is released from the patch into the stratum corneum and epidermis as drug accumulates within these layers to form a depot. Afterwards, fentanyl is released into the systemic circulation slowly from small blood vessels within the dermis. Fentanyl exhibits wide tissue distribution to various organ systems, which is indicative of a high extravascular volume of distribution (3–8 L/kg). It takes about 17 hours to reach steady-state plasma concentrations after initiating fentanyl patch therapy.18
Following patch application, drug release occurs at a constant rate for up to 72 hours.17 Neither the local blood supply nor the anatomical site affectively change the rate or extent of drug absorption. It may take 34 to 38 hours to reach a maximum serum concentration of fentanyl after patch application. Steady-state serum concentrations are typically reached by day 6 and can be maintained with regularly scheduled patch changes at 72-hour intervals.
Fentanyl is primarily metabolized by CYP 3A4 isoenzymes by N-dealkylation to norfentanyl, an inactive metabolite.19 As previously described with methadone, potent inhibitors or inducers of 3A4 isoenzymes may significantly affect the metabolism of fentanyl.
CASES
Please note that the following cases may lend themselves to several therapeutic and clinical adjustment(s); however, discussion points will focus on opioid therapy pharmacokinetics and therapeutic interventions.
CASE 1: OXYCODONE SR AND ABERRANT BEHAVIOR
YE is a 47-year-old white male who recently moved to a new geographic location. He was referred to a new primary care provider (PCP) from his previous doctor. The referral indicated that the patient has chronic low back pain, is currently unemployed, and is seeking social security disability (SSI).
Problem List: DJD of Lumbar spine (L)4, L5, Sacral (S)1, status post (S/P) low back surgery for a herniated disc 6 years ago; magnetic resonance imaging (MRI) report finds no abnormalities other than mild arthritis and surgical evidence; no scar tissue present; hypertension controlled with medication; hyperchosterolemia; aberrant behavior; GERD; and history of substance abuse including heroin. The patient denies use of any recreational drugs at this time, including alcohol.
Current Medications:
OxyContin® 160 mg (2 × 80 mg tablets) PO q12h
oxycodone/acetaminophen/325, take 2 PO q4h prn pain
gabapentin 800 mg PO tid
simvastatin 40 mg PO qam
lisinopril 40 mg PO qam
ibuprofen 600 mg PO qid prn
omeprazole 20 mg PO qam
Height = 71 inches
Weight = 165 lbs
YE presents to his new PCP Dr. P. for a full exam and workup. The patient has 2 days left of OxyContin® and no more oxycodone/acetaminophen for breakthrough pain. He is requesting new prescriptions for these medications ASAP because he is fearful of pain and withdrawal symptoms should he not receive these medications promptly.
Dr. P. evaluates the patient and explains that all of his pain patients receiving chronic opioid therapy must agree to sign a “controlled substance treatment agreement,” which includes consent for random urine drug screens (UDS) and serum analyses. Any prescriptions on the first visit will require a baseline UDS. YE reluctantly agreed to these terms and provided a urine specimen and blood for a free serum oxycodone level. The initial UDS was positive for cannabinoids. The serum oxycodone analysis was expected to take approximately 10 days for the results.
Dr. P. contacted his collaborating pharmacist to discuss the case, as his policy is that if a patient is illegally using marijuana, opioids are not an option. The pharmacist is quick to point out that the UDS screen is an enzyme test and that it is possible to see a false positive from proton pump inhibitors, in this case, omeprazole.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree