PAIN PATHWAYS
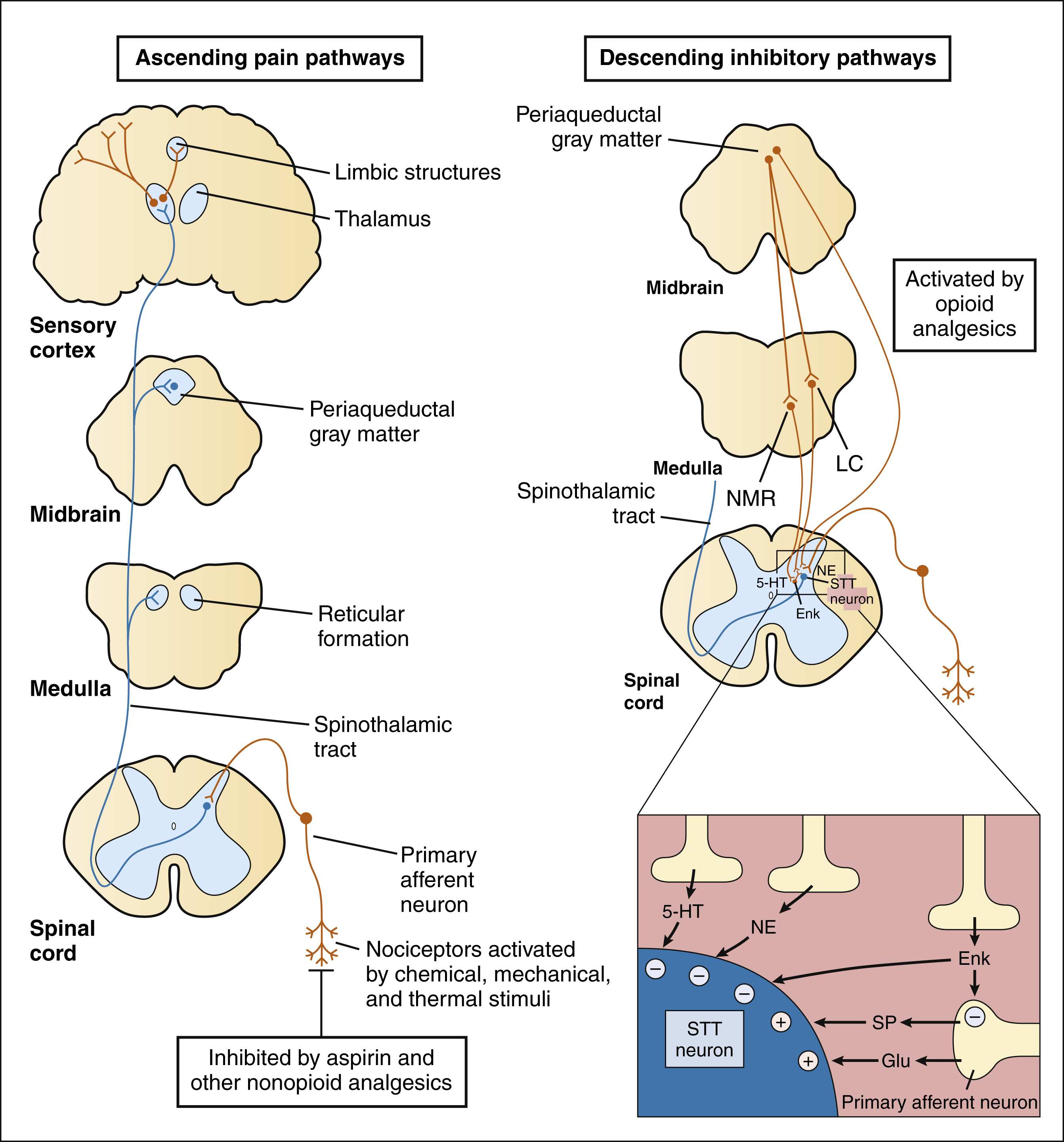
Exposure to a noxious stimulus activates nociceptors on the peripheral free nerve endings of primary afferent neurons. The cell bodies of these neurons sit alongside the spinal cord in the dorsal root ganglia and send one axon to the periphery and one to the dorsal horn of the spinal cord. With noxious stimulation, substance P, glutamate, and other excitatory neurotransmitters are released from the central terminations of the primary afferent fibers onto neurons of the spinal cord. Many of these terminals synapse directly on spinothalamic tract neurons in the dorsal horn, which send long fibers up the contralateral side of the spinal cord to transmit pain impulses via ascending pain pathways to the medulla, midbrain, thalamus, limbic structures, and cortex.
As shown in Box 23–1, the primary afferent fibers transmitting nociceptive information are Aδ fibers and C fibers, which are responsible for sharp pain and dull pain, respectively. Spinal reflexes activated by these fibers can lead to withdrawal from a noxious stimulus before pain is perceived by higher structures. Ascending pain pathways consist of two main anatomical-functional projections: the sensory-discriminative component, to the cerebral cortex, and the motivational-affective component, to the limbic cortex. Projections to the sensory cortex alert an individual to the presence and anatomic location of pain, whereas projections to limbic structures (e.g., the amygdala) enable the individual to experience discomfort, suffering, and other emotional reactions to pain.
The activation of spinothalamic neurons in the spinal cord are modulated by descending inhibitory pathways from the midbrain and by sensory Aβ fibers arising in peripheral tissues. These two systems constitute the neurologic basis of the gate-control hypothesis. According to this hypothesis, pain transmission by spinothalamic neurons can be modulated, or gated, by the inhibitory activity of other types of large fibers impinging on them. The activation of spinothalamic neurons is also inhibited by peripheral Aβ sensory fibers that stimulate the release of met-enkephalin from spinal cord interneurons. The Aβ fibers are thought to also mediate the analgesic effect produced by several types of tissue stimulation, including acupuncture and transcutaneous electrical nerve stimulation (TENS). These mechanisms explain the pain relief that may be produced by simply rubbing or massaging a mildly injured tissue.
The descending inhibitory pathways arise from periaqueductal gray (PAG) in the midbrain and they project to medullary nuclei that transmit impulses to the spinal cord (see Box 23–1). The medullary neurons include serotonergic nerves arising in the nucleus magnus raphae (NMR) and noradrenergic nerves arising in the locus ceruleus (LC). When these nerves release serotonin and norepinephrine in the spinal cord, they inhibit dorsal spinal neurons that transmit pain impulses to supraspinal sites. Nerve fibers from the PAG also activate spinal interneurons that release an endogenous opioid peptide, met-enkephalin. The enkephalins act presynaptically to decrease the release of pain transmitters from the central terminations of primary afferent neurons. They also act on postsynaptic receptors on spinothalamic tract neurons in the spinal cord to decrease the rostral transmission of the pain signal. Opioid analgesics activate the descending PAG, NMR, and LC neuronal pathways, and they also directly activate opioid receptors in the spinal cord.
OPIOID PEPTIDES AND RECEPTORS
Since ancient times, opium, the raw extract of the poppy plant, Papaver somniferum, has been used for the treatment of pain and diarrhea. During the 19th century, morphine was isolated from opium, and its pharmacologic effects were characterized. Later, specific sites in CNS tissue were discovered that bound morphine and other opioid agonists. The presence of stereoselective receptors for morphine in brain tissue indicated the likelihood of an endogenous ligand for these receptors, and this eventually led to the discovery of the three major families of endogenous opioid peptides: enkephalins, β-endorphins, and dynorphins.
The opioid peptides are derived from larger precursor proteins that are widely distributed in the brain. Endorphins and dynorphins are large peptides, whereas the two types of enkephalins are small pentapeptides containing Tyr-Gly-Gly-Phe-Met/Leu. Hence, the two types of enkephalins are called met-enkephalin and leu-enkephalin.
The enkephalins are released from neurons throughout the pain axis, including those in the PAG, medulla, and spinal cord. Enkephalins activate opioid receptors in these areas and thereby block the transmission of pain impulses. The enkephalins appear to act as neuromodulators in that they exert a long-acting inhibitory effect on the release of excitatory neurotransmitters by several neurons.
Opioid agonists mediate their effects at three types of opioid receptors; mu (μ) opioid receptors, delta (δ) opioid receptors, or kappa (к) opioid receptors. Most of the clinically useful opioid analgesics, however, have preferential or strong selectivity for mu opioid receptors. Some of the mixed opioid agonist-antagonist agents have kappa opioid receptor selectivity, but attempts to develop useful opioid analgesics selective for delta receptors have not been successful.
BOX 23–2 THE CASE OF A PAINFUL POX
CASE PRESENTATION
A retired pharmacology professor presents to his primary care physician complaining of an itching and painful rash on his abdomen that is distributed like a band across both sides. The physician asks him whether he had chicken pox as a child, to which the professor replies that he did. His physician diagnoses him with shingles and tells him the rash will go away in about a week. Later that same month, the professor returns to the doctor’s office complaining of pain on his stomach when his clothes rub against it, and sometimes when he is lying in bed. The doctor tells him he has postherpetic neuralgia and prescribes tramadol for the pain.
CASE DISCUSSION
Postherpetic neuralgia is a painful condition that develops after a case of the shingles. Shingles is the name given to a rash that develops from reactivation of chicken pox, the herpes varicella-zoster virus that lay dormant in the cell bodies of the dorsal root ganglia. It is estimated that in the United States nearly 1 million cases of shingles occur every year, mostly in the elderly with 40% to 50% of cases occurring in people that are 60 years of age and older. This may be due to waning immunological defenses or activation by drugs or other disease states. For reasons that are unclear, a number of these cases convert to postherpetic neuralgia. Drug treatment for this neuropathic pain condition includes antidepressants, opioids, and pregabalin. Tramadol is a unique dual-acting opioid agent and acts as an agonist at mu opioid receptors and inhibits the neuronal reuptake of serotonin and norepinephrine. It has shown effectiveness in a number of neuropathic pain states including postherpetic neuralgia.
OPIOID DRUGS
Classification
The opioid drugs can be classified as full agonists, mixed agonist-antagonists, or pure antagonists.
Based on their maximal clinical effectiveness, the full agonists can be characterized as strong or moderate agonists. In experimental pain models, all of the full agonists exert a maximal analgesic effect. In humans, the strong opioid agonists are well tolerated when they are given in a dosage sufficient to relieve severe pain. The moderate opioid agonists, however, will cause intolerable adverse effects if they are given in a dosage sufficient to alleviate severe pain. For this reason, the moderate opioid agonists are administered in submaximal doses to treat moderate to mild pain, and they are usually formulated in combination with NSAIDs to enhance their clinical effectiveness.
The mixed opioid agonist-antagonists are analgesic drugs that have varying combinations of agonist, partial agonist, and antagonist activity and varying degrees of affinity for the different opioid receptor types.
The opioid antagonists have no analgesic effects. They are used to counteract the adverse effects of opioids taken in overdose and for the treatment of drug dependence.
Drug Properties
Mechanism of Action
The opioid receptors are prominent members of the G protein–coupled receptor superfamily. Activation of opioid receptors leads to inhibition of adenylyl cyclase and a decrease in the concentration of cyclic adenosine monophosphate, an increase in K+ conductance, and a decrease in Ca2+ conductance (Fig. 23–1). The activated Gαi subunit of the G protein directly inhibits the adenylyl cyclase enzyme, and the Gβγ subunits are thought to mediate the changes at the Ca2+ and K+ channels. These actions cause both presynaptic inhibition of neurotransmitter release from the central terminations of small-diameter primary afferent fibers and postsynaptic inhibition of membrane depolarization of dorsal horn nociceptive neurons.
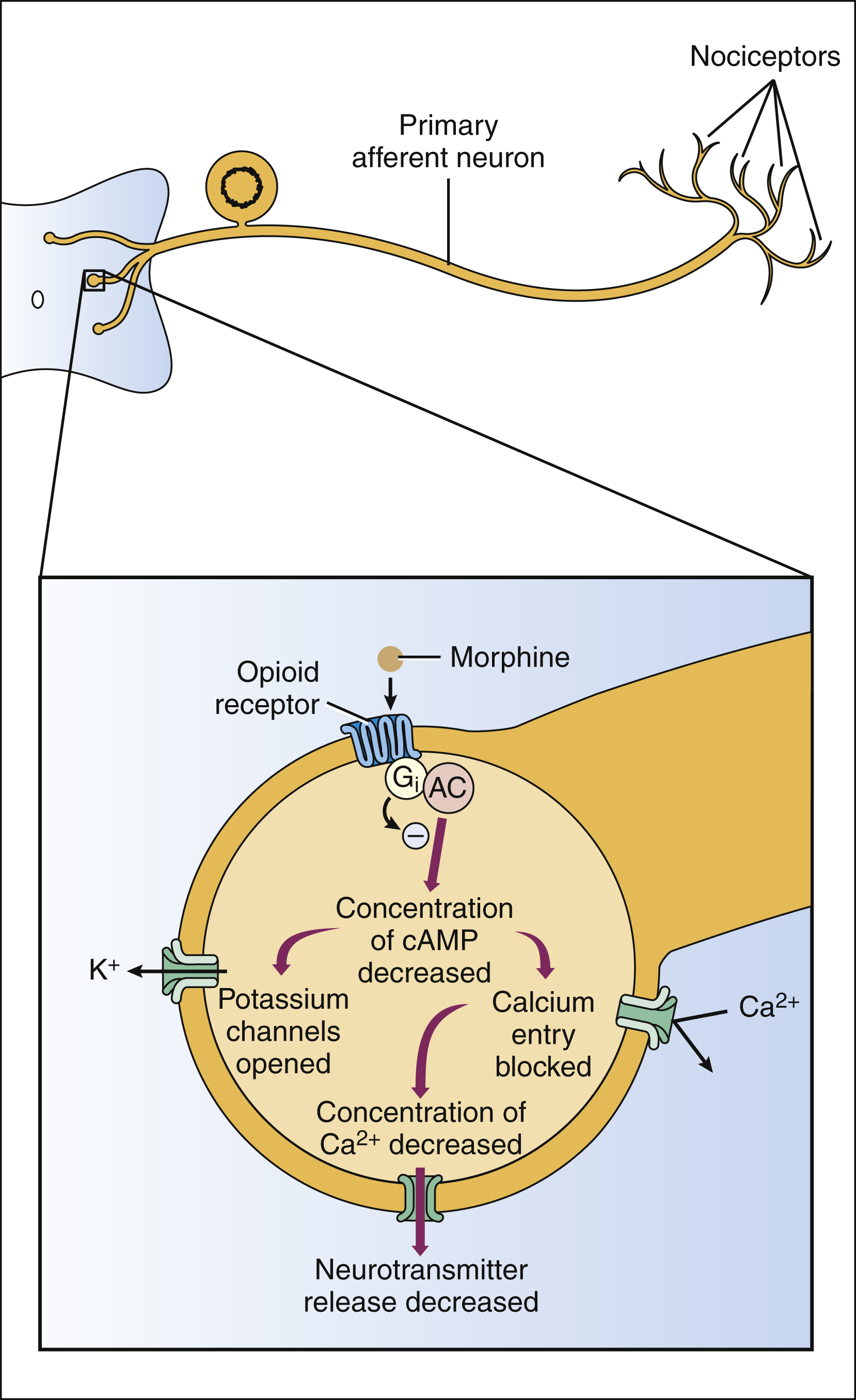
Figure 23–1 Mechanisms of opioid action in the spinal cord. Morphine and other opioid agonists activate presynaptic mu, delta, or kappa opioid receptors on primary afferent neurons. These receptors are coupled negatively to adenylyl cyclase (AC) via G proteins (Gαi). Inhibition of cyclic adenosine monophosphate (cAMP) formation leads to opening of potassium channels and closing of calcium channels. Gβγ subunits may also participate in the modulation of ion channels. Potassium efflux causes membrane hyperpolarization. The closing of calcium channels inhibits the release of neurotransmitters, such as substance P.
Pharmacologic Effects
CENTRAL NERVOUS SYSTEM
Morphine acts in the CNS to produce analgesia, sedation, euphoria or dysphoria, miosis, nausea, vomiting, respiratory depression, and inhibition of the cough reflex (Table 23–1).
TABLE 23–1 Major Pharmacologic Effects of Opioid Agonists
| CNS Effects |
| Analgesia |
| Dysphoria or euphoria |
| Inhibition of cough reflex |
| Miosis |
| Physical dependence |
| Respiratory depression |
| Sedation |
| Cardiovascular Effects |
| Decreased myocardial oxygen demand |
| Vasodilation and hypotension |
| Gastrointestinal and Biliary Effects |
| Constipation (increased intestinal smooth muscle tone) |
| Increased biliary sphincter tone and pressure |
| Nausea and vomiting (via CNS action) |
| Genitourinary Effects |
| Increased bladder sphincter tone |
| Prolongation of labor |
| Urinary retention |
| Neuroendocrine System Effects |
| Inhibition of release of luteinizing hormone |
| Stimulation of release of antidiuretic hormone and prolactin |
| Immune System Effects |
| Suppression of function of natural killer cells |
| Dermal Effects |
| Flushing |
| Pruritus |
| Urticaria (hives) or other rash |
CNS = central nervous system.
Analgesia is produced by activation of opioid receptors in the spinal cord and at several supraspinal levels, as illustrated in Box 23–1. Sedation and euphoria can be caused by effects on midbrain dopaminergic, serotonergic, and noradrenergic nuclei. Surprisingly, many patients experience dysphoria after administration of opioids. Miosis or constricted pupils is produced by the direct stimulation of the Edinger-Westphal nucleus of the oculomotor nerve (cranial nerve III), which activates parasympathetic stimulation of the iris sphincter muscle. Because little or no tolerance develops to miosis, this sign can be diagnostic of an opioid overdose.
Codeine and other opioids inhibit the cough reflex at sites in the medulla where this reflex is integrated. The antitussive actions of opioids are discussed in greater detail in Chapter 27.
CARDIOVASCULAR SYSTEM
The most prominent cardiovascular effect of morphine and many other opioids is vasodilation, which is partly caused by histamine release from mast cells in peripheral tissues. Morphine can cause orthostatic hypotension from decreased peripheral resistance and a reduction in baroreceptor reflex activity. In patients with coronary artery disease, the decreased peripheral resistance leads to a reduction of cardiac work and myocardial oxygen demand.
GASTROINTESTINAL, BILIARY, AND GENITOURINARY SYSTEM
Morphine and most other opioids act to increase smooth muscle tone in the gastrointestinal, biliary, and genitourinary systems. In the gastrointestinal tract, increased muscle tone leads to inhibition of peristalsis and causes constipation. For this reason, the opioids are the oldest and most widely used medication for the treatment of diarrhea (see Chapter 28). Unfortunately, chronic pain patients do not appear to become tolerant to the constipating effects of opioids, necessitating a continual need for laxatives and other agents.
Morphine and other opioids also increase the tone of the biliary sphincter (sphincter of Oddi), and can cause an exacerbation of pain in patients with biliary dysfunction or a gall bladder attack. Opioids also increase the tone of the bladder sphincter and can cause urinary retention in some patients. Because the opioid agonist, meperidine, has less pronounced action on smooth muscle, it is the drug of choice for these patients and for the pain associated with labor.
OTHER EFFECTS
Opioids have an effect on neuroendocrine and immunologic function. In the hypothalamus, they stimulate the release of antidiuretic hormone and prolactin and inhibit the release of luteinizing hormone. Opioids also suppress the activity of certain types of lymphocytes, including natural killer cells, and this action may contribute to the high rate of infectious diseases in heroin addicts.
Adverse Effects
The major adverse effect of morphine and other opioids is respiratory depression, which is usually the cause of death in severe overdoses. Opioids reduce the hypercapnic drive (the stimulation of respiratory centers by increased carbon dioxide levels) while producing relatively little effect on the hypoxic drive. Opioids reduce the respiratory tidal volume and rate, causing the rate to fall to three or four breaths per minute after an opioid overdose. As the cerebral circulation is exquisitely sensitive to CO2 levels and responds with an increase in cerebral blood flow, leading to increased intracranial pressure, opioids should not be used in the case of a closed-head injury. The respiratory depressant effects of opioids are rapidly reversed by the intravenous administration of an opioid antagonist such as naloxone (see later text).
By stimulating the chemoreceptor trigger zone in the medulla, the opioids also cause nausea and vomiting. This is seen most often in ambulatory patients as opioids increase the sensitivity of the vestibular organ of the inner ear.
Opioids cause mast cells throughout the body to release histamine, which can cause itching, or pruritus. A flushing reaction, noted by redness and a feeling of warmth over the upper torso, may also occur from histamine release.
Allergic reactions to opioid analgesics are not uncommon. In most cases, however, a patient who is allergic to a particular opioid can use an opioid from a different chemical class. For example, someone who is allergic to codeine will probably not be allergic to propoxyphene or fentanyl.
Tolerance and Physical Dependence
Tolerance is defined as a decrease in initial pharmacologic effect observed following chronic or long-term administration. Repeated administration of an opioid agonist will lead to pharmacodynamic tolerance for both the administered opioid and other opioid analgesics. Tolerance primarily results from down-regulation of opioid receptors. Interestingly, in animal models, the magnitude of tolerance is inversely proportional to the efficacy of the opioid analgesic. This is because at equi-analgesic doses, a more efficacious opioid will occupy a lesser fraction of available opioid receptors than a less efficacious agent. Tolerance develops to most of the effects of opioids but not to miosis and constipation. Although considerable tolerance to respiratory depression occurs, a sufficiently high dose of an opioid can still be fatal to highly opioid tolerant individuals.
Opioid tolerance is usually accompanied by a similar degree of physical dependence. Physical dependence is defined as a physiologic state in which a person’s continued use of a drug is required for his or her well-being. Tolerance and physical dependence appear with many drug classes and represent the establishment of a new equilibrium between the neuron and its environment (neuroadaptation), wherein the neuron becomes less responsive to the drug while requiring continued drug effect to maintain cellular homeostasis. If the chronic drug is abruptly withdrawn, the equilibrium is disturbed and a rebound hyperexcitability occurs owing to the loss of the inhibitory influence of the drug. This produces a withdrawal syndrome, the manifestations of which depend on the particular type of drug (see Chapter 25).
Because opioids demonstrate cross-tolerance, one opioid drug can substitute for another opioid drug and prevent symptoms of withdrawal in a physically dependent person. This is the basis for outpatient treatment of opioid dependence by the use of methadone or buprenorphine (see Chapter 25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree