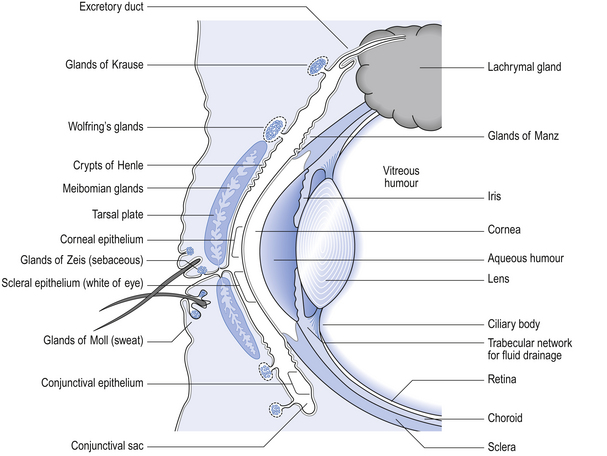
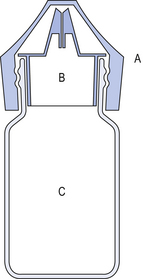
42 The human eye is a remarkable organ and the ability to see is one of our most treasured possessions. Thus, the highest standards are necessary in the compounding of ophthalmic preparations and the greatest care is required in their use. It is necessary that all ophthalmic preparations are sterile and essentially free from foreign particles. These preparations may be categorized as follows: Medicaments contained in ophthalmic products include: Figure 42.1 gives an indication of the relevance of the external structures of the eye and the structure of the eyelids to the application of medication and the wearing of contact lenses (see p.406 also). The components of an eye drop formulation are given below: Multiple-dose eye drops contain an effective antimicrobial preservative system, which is capable of withstanding the test for efficacy of antimicrobial preservatives of the British Pharmacopoeia (BP 2007). This ensures that the eye drops are maintained sterile during use and will not introduce contamination into the eyes being treated. Normal healthy eyes are quite efficient at preventing penetration by microorganisms. Eyes that have damaged epithelia are compromised and may be colonized by microorganisms. This has to be guarded against. The lack of vascularity of the cornea and certain internal structures of the eye make it very susceptible and difficult to treat once infection has been established. Eye drops specifically formulated for use during intraocular surgery should not contain a preservative because of the risk of damage to the internal surfaces of the eye. Diagnostic dyes should preferably be supplied as single-dose preparations. Preservatives which are suitable for a selection of eye drops are given in Box 42.1. The surface activity of benzalkonium chloride may be used to enhance the transcorneal passage of non-lipid-soluble drugs such as carbachol. Care must be taken since the preservative can solubilize the outer oily protective layer of the precorneal film. This film has an internal mucin layer in contact with the corneal and scleral epithelia, a middle aqueous layer and an outer oily layer. The oil prevents excessive aqueous evaporation and protects the inner surface of the lids from constant contact with water. The blink reflex helps maintain the integrity of the precorneal film. For these reasons, it is important not to use benzalkonium chloride to preserve local anaesthetic eye drops which abolish the blink reflex. The combined effect of the two agents causes drying of the eye surface and irritation of the cornea. Where possible, eye drops are made isotonic with lachrymal fluid (approximately equivalent to 0.9% w/v sodium chloride solution). In practice, the eye will tolerate small volumes of eye drops having tonicities in the range equivalent to 0.7–1.5% w/v sodium chloride. Nevertheless it is good practice to adjust the tonicity of hypotonic eye drops by the addition of sodium chloride to bring the solution to the tonicity of the lachrymal fluid. (See Ch. 19 for methods for calculating the amount of sodium chloride required.) Some preparations are themselves hypertonic and so no adjustment should be made. The best compromise is required after considering the following factors: The lachrymal fluid has a pH of 7.2–7.4 and also possesses considerable buffering capacity. Thus a 50 μL eye drop which is weakly buffered will be rapidly neutralized by lachrymal fluid. Where it is possible, very acidic solutions, such as adrenaline acid tartrate or pilocarpine hydrochloride, are buffered to reduce a stinging effect on instillation. Suitable buffers are shown in Box 42.2. The effect of pH on the therapeutic activity of weak bases such as atropine sulphate has already been indicated under the section on pH adjustment. At acid pH, these bases exist in the ionized hydrophilic form. In order to penetrate the cornea, the bases need to be at alkaline pH so that they are in the unionized lipophilic form. Thus at tear pH (7.4) they are able to penetrate the outer lipid layer of the lipid–water–lipid sandwich, which constitutes the physicochemical structure of the cornea. Once inside the epithelium the undissociated free base will partially dissociate. The water-soluble dissociated moiety will then traverse the middle aqueous stromal layer of the cornea. When the dissociated drug reaches the junction of the stroma and the endothelium it will again partially associate, forming the lipid-soluble moiety and thus cross the endothelium. Finally, the drug will dissociate into its water-soluble form and enter the aqueous humour. From here it can diffuse to the iris and the ciliary body which are the sites of its pharmacological action (see Fig. 42.1). Thus, the most effective penetration of the lipophilic–hydrophilic–lipophilic corneal membrane is by active ingredients having both hydrophilic and lipophilic forms. For example, highly water-soluble steroid phosphate esters have poor corneal penetration but the less water-soluble, more lipophilic steroid acetate has much better corneal penetration. Most commercially prepared eye drops are supplied in plastic dropper bottles similar to the illustration in Figure 42.2. Bottles are made of polyethylene or polypropylene and are sterilized by ionizing radiation prior to filling under aseptic conditions with the previously sterilized preparation. Figure 42.2 Plastic eye drop bottle. (A) Rigid plastic cap. (B) Polythene friction plug containing baffle that produces uniform drops. (C) Polythene bottle.
Ophthalmic products
 The formulation, preparation and uses of ophthalmic preparations
The formulation, preparation and uses of ophthalmic preparations
 The packaging and labelling requirements for ophthalmic preparations
The packaging and labelling requirements for ophthalmic preparations
 Advising patients on the use of eye medication and on any adverse effects
Advising patients on the use of eye medication and on any adverse effects
 The anatomy and physiology of the eye in relation to the administration of medication and the wearing of contact lenses
The anatomy and physiology of the eye in relation to the administration of medication and the wearing of contact lenses
 The properties of contact lenses in relation to their physicochemical composition
The properties of contact lenses in relation to their physicochemical composition
 The wearing of and caring for contact lenses and the various products available to facilitate comfort, effectiveness, convenience and safety
The wearing of and caring for contact lenses and the various products available to facilitate comfort, effectiveness, convenience and safety
 The role of antimicrobial preservatives in ophthalmic products
The role of antimicrobial preservatives in ophthalmic products
 Advising patients on the possible adverse effects of concurrent medication and the sensible use of cosmetics when wearing contact lenses
Advising patients on the possible adverse effects of concurrent medication and the sensible use of cosmetics when wearing contact lenses
Introduction
 Eye drops including solutions, emulsions and suspensions of active medicaments for instillation into the conjunctival sac
Eye drops including solutions, emulsions and suspensions of active medicaments for instillation into the conjunctival sac
 Eye lotions for irrigating and cleansing the eye surface, or for impregnating eye dressings
Eye lotions for irrigating and cleansing the eye surface, or for impregnating eye dressings
 Eye ointments, creams and gels containing active ingredient(s) for application to the lid margins and/or conjunctival sac
Eye ointments, creams and gels containing active ingredient(s) for application to the lid margins and/or conjunctival sac
 Contact lens solutions to facilitate the wearing and care of contact lenses
Contact lens solutions to facilitate the wearing and care of contact lenses
 Parenteral products for intracorneal, intravitreous or retrobulbar injection
Parenteral products for intracorneal, intravitreous or retrobulbar injection
 Ophthalmic inserts placed in the conjunctival sac and designed to release active ingredient over a prolonged period
Ophthalmic inserts placed in the conjunctival sac and designed to release active ingredient over a prolonged period
 Anaesthetics used topically in surgical procedures
Anaesthetics used topically in surgical procedures
 Anti-infectives such as antibacterials, antifungals and antivirals
Anti-infectives such as antibacterials, antifungals and antivirals
 Anti-inflammatories such as corticosteroids and antihistamines
Anti-inflammatories such as corticosteroids and antihistamines
 Antiglaucoma agents to reduce intraocular pressure, such as beta-blockers
Antiglaucoma agents to reduce intraocular pressure, such as beta-blockers
 Astringents such as zinc sulphate
Astringents such as zinc sulphate
 Diagnostic agents such as fluorescein which highlight damage to the epithelial tissue
Diagnostic agents such as fluorescein which highlight damage to the epithelial tissue
 Miotics such as pilocarpine which constrict the pupil and contract the ciliary muscle, increasing drainage from the anterior chamber
Miotics such as pilocarpine which constrict the pupil and contract the ciliary muscle, increasing drainage from the anterior chamber
 Mydriatics and cycloplegics such as atropine, which dilate the pupil and paralyse the ciliary muscle and thus facilitate the examination of the interior of the eye.
Mydriatics and cycloplegics such as atropine, which dilate the pupil and paralyse the ciliary muscle and thus facilitate the examination of the interior of the eye.
Anatomy and physiology of the eye
Formulation of eye drops
 Active ingredient(s) to produce desired therapeutic effect
Active ingredient(s) to produce desired therapeutic effect
 Vehicle, usually aqueous but occasionally may be oil
Vehicle, usually aqueous but occasionally may be oil
 Antimicrobial preservative to eliminate any microbial contamination during use and thus maintain sterility; it should not interact adversely with the active ingredient(s)
Antimicrobial preservative to eliminate any microbial contamination during use and thus maintain sterility; it should not interact adversely with the active ingredient(s)
 Adjuvants to adjust tonicity, viscosity or pH in order to increase the ‘comfort’ in use and to increase the stability of the active ingredient(s); they should not interact adversely with other components of the formulation
Adjuvants to adjust tonicity, viscosity or pH in order to increase the ‘comfort’ in use and to increase the stability of the active ingredient(s); they should not interact adversely with other components of the formulation
 Suitable container for administration of eye drops which maintains the preparation in a stable form and protects from contamination during preparation, storage and use.
Suitable container for administration of eye drops which maintains the preparation in a stable form and protects from contamination during preparation, storage and use.
Antimicrobial preservatives
Benzalkonium chloride
Tonicity
Viscosity enhancers
pH adjustment
 The pH offering best stability during preparation and storage
The pH offering best stability during preparation and storage
 The pH offering the best therapeutic activity
The pH offering the best therapeutic activity
Bioavailability
Containers for eye drops
Plastic bottles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ophthalmic products
Only gold members can continue reading. Log In or Register to continue