(1)
Department of Pathology, Rutgers-New Jersey Medical School, Newark, NJ, USA
Keywords
MüllerianWolffianEmbryologyVulvaHistologyLabia majoraLabia minoraVestibuleBartholin’s glandsClitorisPerineumMons pubisHymenSkene’s ductSkene’s glandsVaginaHistologyCervixHistologyEndocervixExocervixTransformation zoneEndometriumHistologyEndometrial datingMyometriumHistologyFallopian tubeHistologyOvaryHistologyPlacentaHistology2.1 Embryology
An in-depth discussion of embryology and anomalies of the female genital tract is beyond the scope of this text, and interested readers are referred to embryology texts; however, a brief review is in order. In the early first trimester, the external genitalia are not differentiated towards either gender. Both the Müllerian (paramesonephric) and Wolffian (mesonephric) ducts are present in parallel. Genetics determine which duct develops and which regresses. For XX individuals, the Müllerian ducts continue to develop, and the Wolffian ducts regress. Müllerian duct development relies on a number of genes in addition to absence of anti-Müllerian hormone (Müllerian inhibiting substance, MIS) [1], and the differentiation into a female is not merely becoming “not male.” Knock-out mice missing a variety of these female-determining genes have a variety of genital anomalies [1]. Differentiation of the embryo begins at about 8 weeks. The external genitalia and lower third of the vagina are formed by the urogenital sinus. The upper two thirds of the vagina, cervix, uterus, and fallopian tubes are formed by the fusion of the two Müllerian (paramesonephric) ducts. After fusion of the Müllerian ducts, the septum between them dissolves. When the urogenital sinus meets the Müllerian ducts, a vaginal plate is formed which subsequently canalizes, forming the patent and lined vagina. In a female, the Wolffian (mesonephric) ducts regress, but remnants may remain and be identified later in life.
The ovaries are indifferent in early embryonic life as well. At about 8 weeks of gestational age, the gonads can be reliably distinguished. This histologic distinction can be extremely important to make when examining an immature fetus from an unsuccessful or terminated pregnancy. Inexperienced clinicians and pathologists tend to mistake the external genitalia of late first/early second trimester female fetuses as male due to the prominence of the clitoris and not looking behind it to see the labia and patent vaginal opening rather than scrotum. Histopathology of the gonads can provide the gonadal gender (Fig. 2.1a, b). Migration of germ cells occurs along the midline along the dorsal mesentery of the hindgut [2], populating the ovaries, which are formed from the gonadal ridges [3]. It is because of this pattern of migration that germ cell neoplasms can occur anywhere in the body along the midline. No additional oogonia develop after birth, and some degenerate prior to birth, the rest enlarging prior to birth into primary oocytes, surrounded by a single flat layer of follicular cells forming the primordial follicle (Fig. 2.2). Therefore, a female is born with all the two to four million oocytes she will ever have. The maternal hormones may persist in the female infant, leading to cystic follicles (Fig. 2.3), which eventually regress in childhood until puberty. The XO fetus may occasionally show a streak gonad devoid of germ cells at birth, but germ cell loss may occur later (Fig. 2.4).
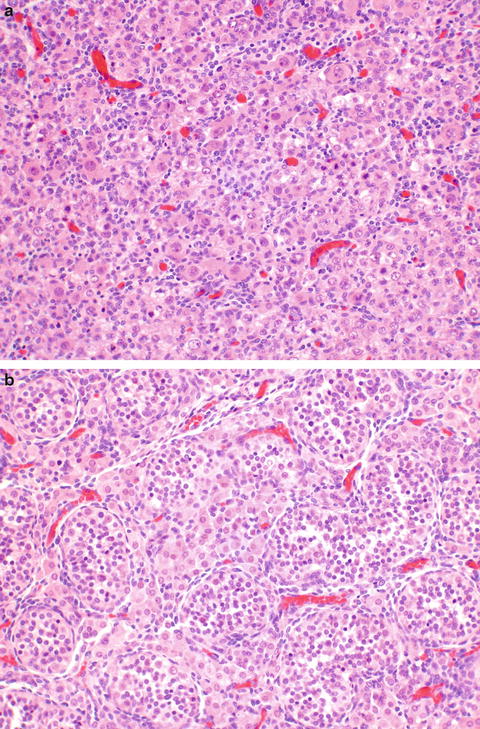
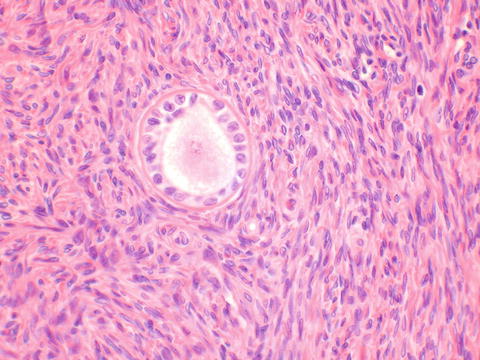
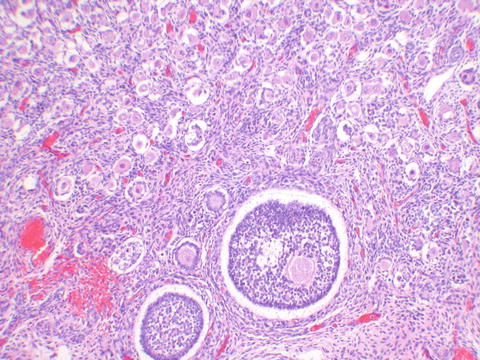
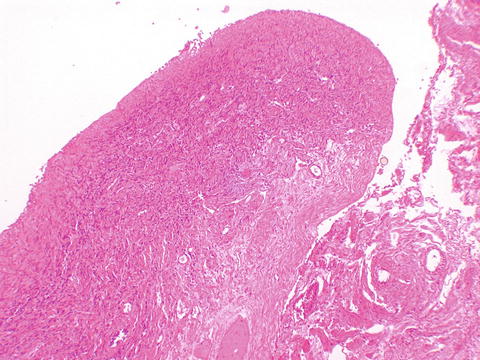

Fig. 2.1
Fetal gonads. The fetal ovary (a) shows diffuse distribution of germ cells. The fetal testis (b) shows distinct tubules containing Sertoli and germ cells, with intervening Leydig cells

Fig. 2.2
The primordial follicle is composed of the ovum surrounded by a single layer of supporting follicular cells

Fig. 2.3
The newborn ovary shows fewer primordial follicles than the fetal ovary, and follicular development as seen at the bottom of the image is a reflection of maternal hormonal effect

Fig. 2.4
Streak ovary devoid of germ cells
Aside from agenesis or hypoplasia, many of the anomalies of the female genital tract can be explained by defects in canalization of the urogenital sinus, or defects in either fusion of the Müllerian ducts or later dissolution of the intervening septum.
2.2 Histology of the Vulva
2.2.1 Labia Majora
The labia majora are similar to skin elsewhere on the body and are lined by a keratinized stratified squamous epithelium (Fig. 2.5). The dermis is less delineable into papillary and reticular dermis than skin elsewhere on the body, which is the basis of the modification of Clark’s levels used for skin to vulvar Chung’s levels for evaluating melanoma [4]. The labia majora contain hair follicles, apocrine, and eccrine glands confined to the outer portion of the labia majora only, and sebaceous glands in both outer and inner portions (Figs. 2.6, 2.7, and 2.8).
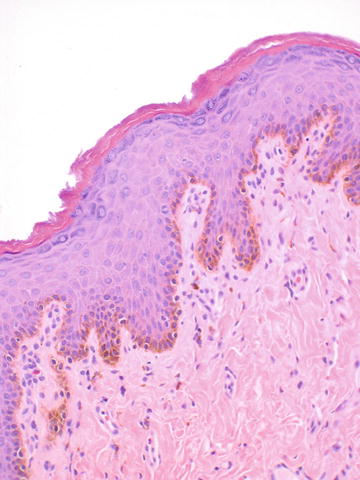
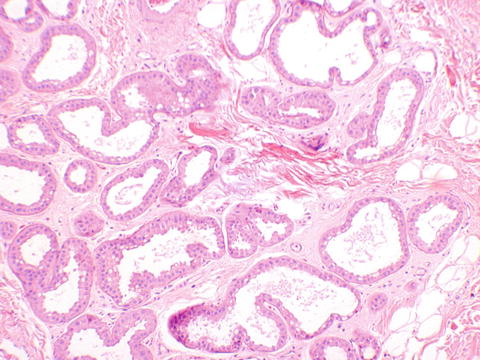
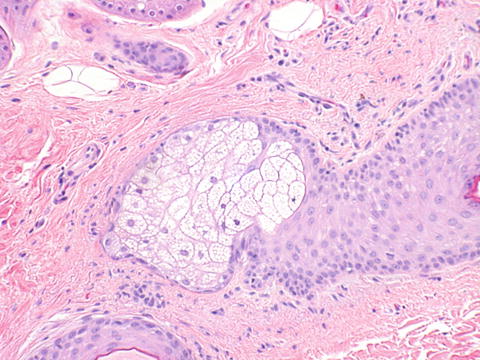
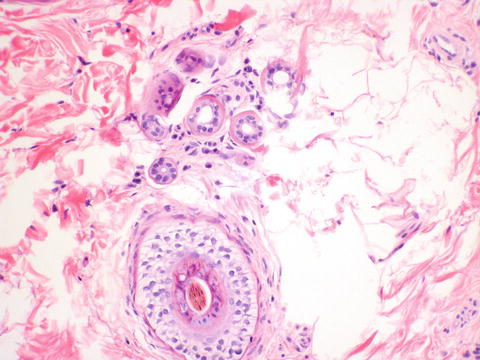

Fig. 2.5
Labia majora lined by keratinized stratified squamous epithelium. The basal pigmentation seen here corresponds to clinically appreciable pigmented skin

Fig. 2.6
Labia majora. Apocrine glands showing abundant eosinophilic cytoplasm

Fig. 2.7
Labia majora. A sebaceous gland is seen

Fig. 2.8
Labia majora. A hair follicle is present at the bottom of the image, with eccrine glands above
Labia minora—The labia minor are lined by squamous epithelium with a thin keratin layer outside, none inside, no hair, and fewer glands than the labia majora. These glands are comprised of sebaceous glands, with no apocrine or eccrine glands, hair follicles, or fat in the dermis. The dermis contains collagen and elastic fibers, blood vessels, and nerves.
Vestibule—The vestibule is the area above Hart’s line, external to the hymen. Sebaceous glands end external to Hart’s line, and there are generally no sweat glands in the vestibule. Minor vestibular glands comprised of acini lined by mucinous columnar epithelium may be present. The vestibule contains the openings of Bartholin’s ducts, vagina, and urethra and is lined by a non-keratinized stratified squamous epithelium.
Bartholin’s glands—The Bartholin’s glands contain three types of epithelium. The glands are composed of acini lined by mucinous columnar epithelium. This merges in the ducts with a transitional epithelium and becomes squamous epithelium at the ostia which open onto the 4 o’clock and 8 o’clock positions of the vestibule (Fig. 2.9).
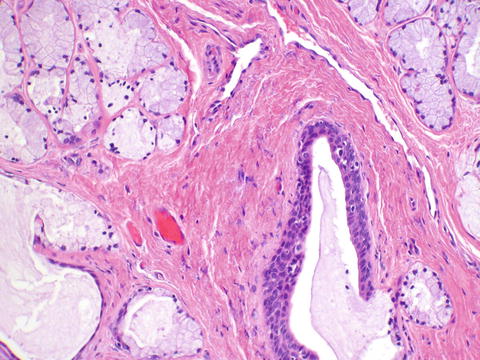

Fig. 2.9
Bartholin’s glands show mucinous acini. A transitional epithelial-lined duct is seen at the bottom right
Clitoris—The clitoris is lined by keratinized stratified squamous epithelium, without dermal appendages. Erectile tissue is abundant beneath the epithelium and is composed of abundant vascular spaces (Fig. 2.10).
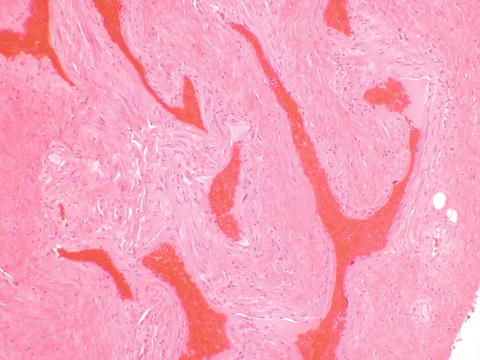

Fig. 2.10
Erectile tissue of the clitoris, containing numerous vascular spaces
Perineum—The perineum is lined by keratinized stratified squamous epithelium. The perineum contains apocrine and mammary-like glands (Fig. 2.11). It used to be thought that there was accessory breast tissue along the milk line. This is now recognized as being anogenital mammary glands, which can also be present at the interlabial sulcus. These glands can give rise to neoplasms similar to those seen in the breast.
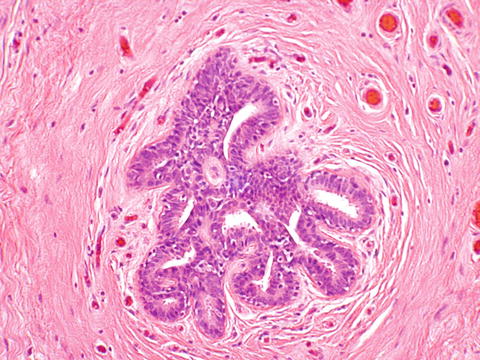

Fig. 2.11
Mammary-like tissue of the vulva showing a ductal structure similar to breast
Mons pubis—The mons pubis is a fat pad covered by hair-bearing skin.
Hymen—The non-keratinized squamous epithelium of the hymen covers a loose fibroelastic tissue.
Skene’s ducts and glands—The Skene’s glands are composed of mucinous columnar epithelium which drains via transitional ducts out on either side of the urethra, where the epithelium blends with the squamous epithelium of the vestibule. It is also thought that branches of the duct drain into the urethra. The Skene’s glands are considered analogous to the male prostate.
2.3 Histology of the Vagina
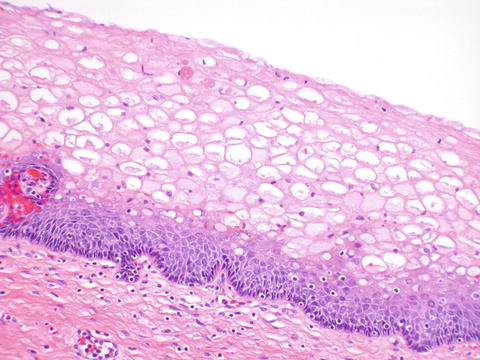
The vagina is lined by non-keratinized stratified squamous epithelium. During reproductive life, the epithelium is highly glycogenated due to the effect of estrogen (Fig. 2.12).
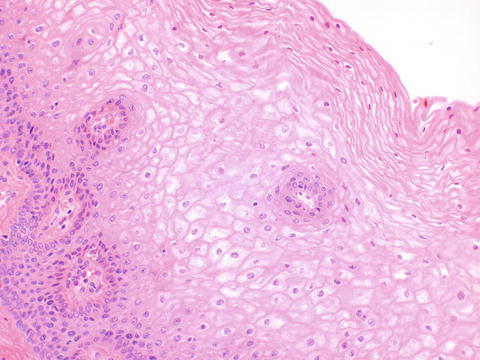

Fig. 2.12
The vagina is lined by non-keratized stratified squamous epithelium containing abundant glycogen during reproductive life
2.4 Histology of the Cervix
2.4.1 Exocervix
The exocervix is lined by non-keratinized stratified squamous epithelium. The cells show an orderly maturation from the basal layer up to the surface, which shows impaired maturation when intraepithelial neoplasia is present. During reproductive life, the presence of estrogen leads to abundant glycogenation of the cells, which should not be mistaken for koilocytes in the absence of nuclear atypia (Fig. 2.13). Persistence of maternal hormones leads to similar glycogenated cervical epithelium in the neonate; however, in the child and menopausal woman, lack of estrogen leads to a more atrophic epithelium. With atrophy there is decreased glycogen in the cells, and the maturation from basal layer to surface is much decreased. This lack of maturation (Fig. 2.14) should not be confused with intraepithelial neoplasia. A Ki-67 immunostain (Fig. 2.15) can be used in difficult cases, because normal epithelium, including atrophic epithelium, will stain only in the parabasal layer, while neoplastic epithelium will stain up to the surface with this proliferation marker.