USUAL PROSTATE ADENOCARCINOMA WITH NEUROENDOCRINE DIFFERENTIATION
In the early 1970s, Azzopardi and Evans4 recognized the presence of argentaffin cells within normal prostatic adenocarcinoma. Immunohistochemically, usual adenocarcinoma of the prostate demonstrates scattered NE cells in 10% to 100% of cases, in part depending on the number of slides studied and the number of antibodies used (Fig. 12.1).7,12,16,17
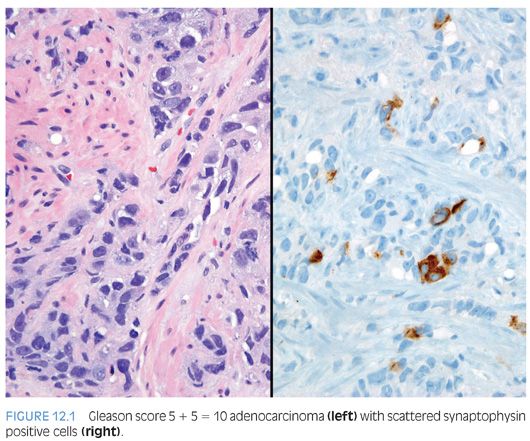
In these cases, prostate-specific antigen (PSA) is positive in the usual adenocarcinoma but variably positive in the NE cells.18 It is controversial whether NE differentiation in typical adenocarcinomas worsens prognosis. In some studies suggesting a correlation, the prognostic relationship was weak and not sufficient to be useful clinically.1,19–22 Most of the studies have shown no effect of NE differentiation on outcome, including one study each analyzing NE differentiation in prostate cancer on needle biopsy and transurethral resection of the prostate (TURP).23–34
Currently, as the clinical significance remains uncertain, it is not recommended to routinely employ immunohistochemical stains to detect any NE differentiation in an otherwise morphologically typical primary adenocarcinoma of the prostate.
ADENOCARCINOMA WITH PANETH CELL-LIKE NEUROENDOCRINE DIFFERENTIATION
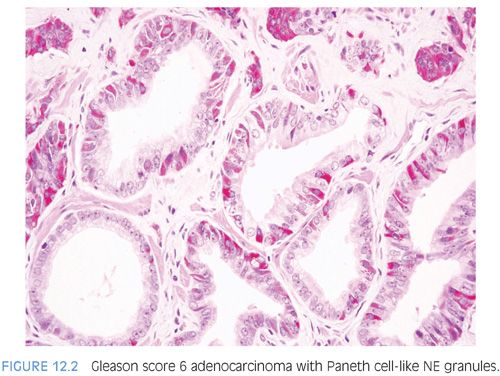
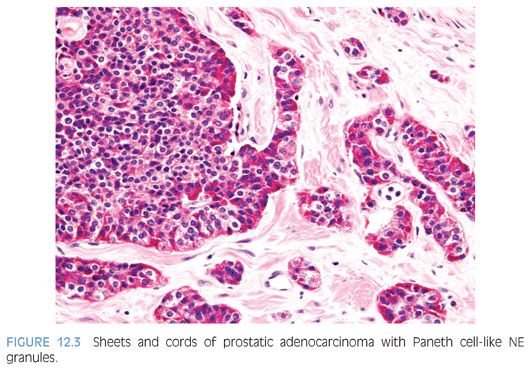
The term Paneth cell-like change has been used to describe distinctive eosinophilic NE cells (eFig. 12.1).35 Paneth cell-like NE differentiation in prostatic adenocarcinoma can be seen as either patchy isolated cells or diffusely involving glands or nests.36,37 These Paneth cell-like cells may be present in well-formed glands of Gleason pattern 3 (Fig. 12.2) but also can be present in cords of cells with bland cytology, where strictly applying the Gleason grading system, one would assign a Gleason pattern 5 (Fig. 12.3, eFigs. 12.2 to 12.7). Although by the Gleason system areas of Paneth cell-like NE differentiation may be graded as pattern 5, their bland cytology, typically limited nature, and frequent association with lower grade conventional adenocarcinoma raise questions as to whether this unique histology should not be diagnosed as high grade. Of 16 radical prostatectomy specimens with Paneth cell-like NE cells lacking glandular differentiation, there was organ-confined cancer in 62.5% of cases, only 4 cases with seminal vesicle invasion, and none with pelvic lymph node metastases. The postoperative course was also favorable with an over 90% actuarial PSA progression-free risk at 5 years. The prognosis seemed to be driven by conventional parameters independent of NE differentiation. The only two patients who progressed after radical prostatectomy had Gleason score 7 conventional cancer with extraprostatic extension and seminal vesicle invasion, with one also having ductal differentiation and positive margins. In cases in which the entire tumor is composed of Paneth cell-like cells and areas of the tumor lack glandular differentiation, it is questionable whether these tumors should be assigned a Gleason score. A comment could be provided as to the generally favorable prognosis of this morphologic variant of adenocarcinoma of the prostate based on the limited data available.36 However, the data on the prognostic significance of Paneth cell-like differentiation is still limited, and we have seen anecdotal cases where such a tumor progressed to metastatic disease with small cell carcinoma.
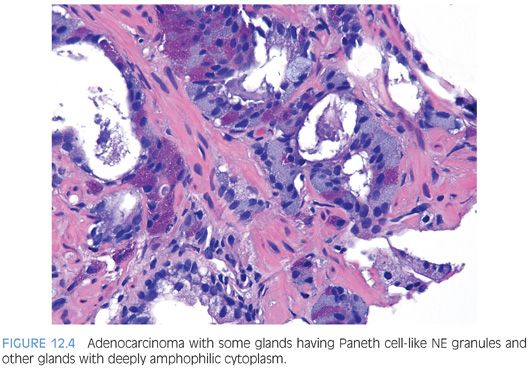
In some cases, one can see a spectrum of Paneth cell-like cells with eosinophilic granules adjacent to identical cells with deeply amphophilic cytoplasm lacking granules, with both cell types labeling diffusely with NE markers (Figs. 12.4 and 12.5). Uncommonly, cancers may only consist of cords of cells with bland cytology and only amphophilic cytoplasm, either with rare or absence of the characteristic eosinophilic granules (Fig. 12.6). These cells are also diffusely positive for NE markers. These cells with amphophilic cytoplasm arranged in cords with bland cytology, typically in a very limited focus, are typically associated with other cells showing Paneth cell-like changes and should be considered a variant of Paneth cell-like change. Both the classic Paneth cell-like changes and this variant may not express prostate markers, possibly given that their cytoplasm is replaced by NE granules. The key to recognizing these cases is first to note the architectural pattern of nests and cords in a small focus. Secondly, these tumors have deeply amphophilic cytoplasm with careful search in most cases, revealing rare Paneth cell-like eosinophilic granules. Finally, the preceding finding in combination with either no prominent nucleoli or rare visible nucleoli may prompt immunohistochemical staining for NE markers.
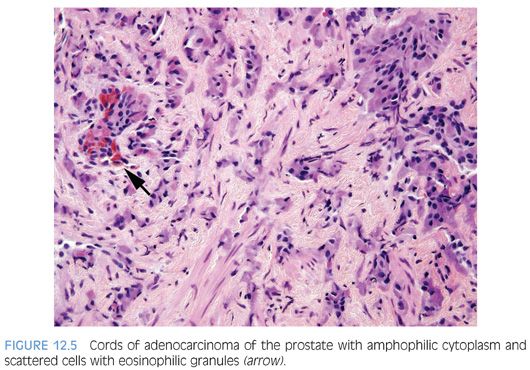
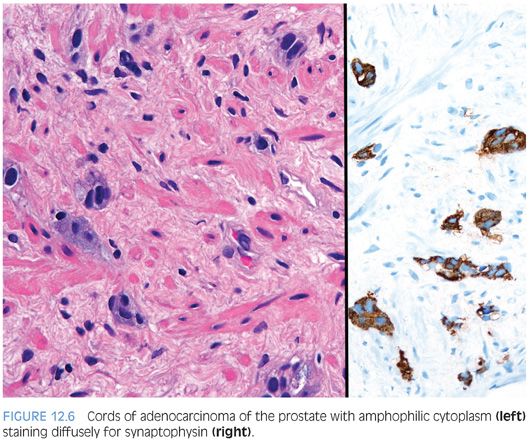
Currently, as the clinical significance is incompletely understood, one may employ immunohistochemical stains to confirm NE differentiation in the eosinophilic (Paneth cell-like) and amphophilic cells. The term adenocarcinoma with Paneth cell-like NE differentiation should be used. Additionally, a comment may be made that in the absence of prior ADT, Gleason grading of areas showing Paneth cell-like or amphophilic NE change in areas without glandular differentiation may not be applicable.
CARCINOID TUMOR
True carcinoid tumors of the prostate are extremely rare. In order to diagnose a carcinoid of the prostate and distinguish it from a prostate adenocarcinoma with carcinoid-like features, the following features should be present: (a) not closely associated with concomitant adenocarcinoma of the prostate, (b) immunohistochemically positive for NE markers and negative for PSA, and (c) originating in the prostatic parenchyma. Of the cases in the literature, there are only five cases that satisfy this definition.38–41 Some of the older reported cases of carcinoid tumor of the prostate predate the use of IHC and cannot be verified. One case based on the illustration provided is a urethral carcinoid as opposed to prostatic in origin.42 The reports by Slater43 in a 69-year-old and by Tash et al.44 in a 38-year-old male may be carcinoids, yet immunohistochemical stains for PSA or any other prostatic marker were not reported. Similarly, in the study by Wasserstein and Goldman,45 no IHC was performed. Murali et al.46 illustrate images of two prostatic carcinoids in their review article, yet no details are provided about the cases. Turbat-Herrera et al.47 reported a “prostatic carcinoid” that was negative for PSA, yet in contrast to carcinoids, only 2+ scattered synaptophysin-positive cells were present and the tumor had diffuse prominent nucleoli. The prostatic carcinoid reported by Egan et al.48 had “intraductal carcinoid” and was admixed with usual prostate adenocarcinoma and most likely represents the recently described phenomenon of “small cell-like change in high-grade prostatic intraepithelial neoplasia, intraductal carcinoma, and invasive prostatic adenocarcinoma.”49 There are five bona fide cases of prostatic carcinoids. Two cases were in men in their 30s, younger than typically seen with adenocarcinoma of the prostate.38,39 The remaining three cases were in even younger males with multiple endocrine neoplasia (MEN) IIB syndrome.40,41 Patients were 7, 19, and 22 years of age. Although the data is limited, prostatic carcinoids tend to present with locally advanced disease, including some with regional lymph node metastases yet still have a favorable prognosis. It is reasonable for these true carcinoids to grade them in an analogous fashion to those of gastrointestinal tract based on mitotic rates and Ki67 proliferation rates.
Several cases have been reported where a “carcinoid-like” or “carcinoidal” appearance of the tumor with nested architecture and uniform nuclei has been present (eFigs. 12.8 and 12.9). These tumors may on occasion also exhibit immunohistochemical and/or ultrastructural evidence of NE differentiation. Some authors consider immunohistochemical staining with PSA a key discriminator where true prostatic carcinoids are negative and carcinoid-like carcinomas are positive. Most cases reported with carcinoid-like morphology have admixed usual prostate cancer or the carcinoid-like tumor expressed PSA.50–56 Several carcinoid-like prostate cancers appear to be variants of Paneth cell-like NE differentiation with a paucity or absence of eosinophilic granules where PSA may be negative (Fig. 12.7).57 Clinically, carcinoid-like adenocarcinomas have behaved like ordinary prostate carcinomas and in none of these cases has a carcinoid syndrome been present. Prostate-specific acid phosphatase (PSAP) immunoreactivity is not discriminatory in the assessment of whether a tumor is a true carcinoid or adenocarcinoma with carcinoid-like features as even some nonprostatic carcinoid tumors express PSAP.58 Although most carcinoid-like tumors have not produced clinical symptoms, several cases have produced adrenocorticotropic hormone (ACTH) in sufficient quantity to result in Cushing syndrome.52
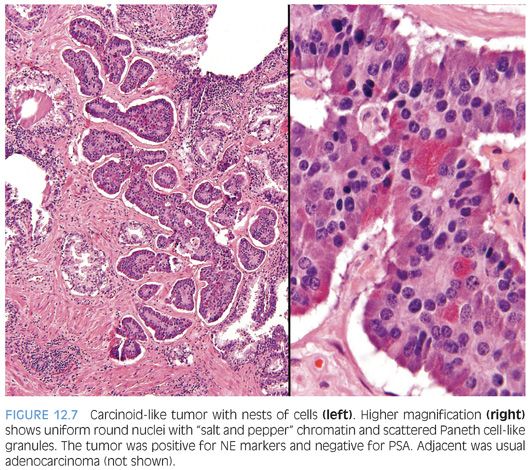
The diagnosis of carcinoid tumor should be made very rarely and strictly, applying the criteria outlined earlier in the definition. In such cases, particularly in younger patients, investigation for stigmata of MEN syndrome should be initiated. Tumors with PSA-negative nests and cords of cells, which are admixed with usual prostate adenocarcinoma, should not be diagnosed as carcinoid tumor because such cases may represent adenocarcinoma with Paneth cell-like NE differentiation or its more subtle variant with cytoplasmic amphophilia.
SMALL CELL CARCINOMA
Small cell carcinoma is a high-grade tumor defined by characteristic nuclear features, including lack of prominent nucleoli, nuclear molding, fragility, and crush artifact. High nuclear to cytoplasmic ratio and indistinct cell borders are characteristic, as is a high mitotic rate and apoptotic bodies (Fig. 12.8, eFigs. 12.10 to 12.15). In resection specimens, as opposed to needle biopsy cores, geographic necrosis may be frequent.
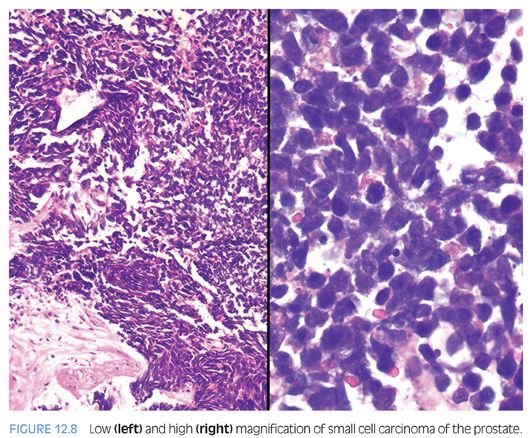
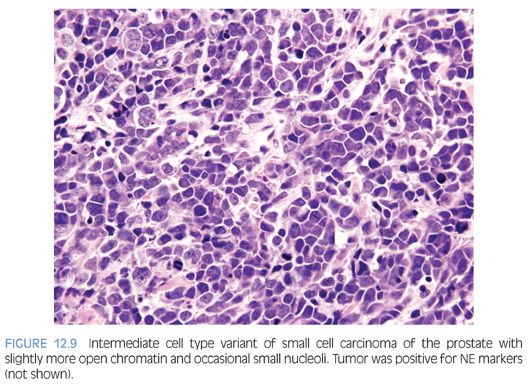
Approximately 40% to 50% of small cell carcinomas have a history of usual prostatic adenocarcinoma. The interval between the diagnosis of small cell carcinoma and prior usual prostatic cancer ranges from 1 to 300 months (median: 25 months).59 Historically, pure small cell carcinoma was seen at initial diagnosis in about 50% to 60% of cases, with the remaining cases admixed with prostate adenocarcinoma (as discussed later). Clinical recognition of the emergence of small cell carcinoma during the progression of the disease is increasing and leading to more frequent biopsies of metastatic sites. Patients with this aggressive disease have frequent visceral metastases and less often paraneoplastic syndromes such as those associated with ectopic ACTH, hypercalcemia, or inappropriate antidiuretic hormone (ADH) production. The diagnosis of small cell carcinoma of the prostate is reached based on morphologic features similar to those found in small cell carcinomas of the lung as defined in the 1999 WHO classification criteria of pulmonary neoplasms.60–62 Morphologic variations of small cell carcinoma include intermediate cell type with slightly more open chromatin and visible small nucleoli seen in about 30% to 40% of cases, which may be beyond that allowable in the strict diagnosis of small cell carcinoma of the lung (Fig. 12.9).59 Less commonly, there is the presence of tumor giant cells and Indian filing.59 Neurosecretory granules have been demonstrated within several prostatic small cell carcinomas. Using immunohistochemical techniques, the small cell component is positive for one or more NE markers (synaptophysin, chromogranin, CD56) in almost 90% of cases.59–61 PSA and other prostatic markers such as P501s are positive in about 17% to 25% of cases, although often very focally.59–61 In 24% to 35% of cases, positivity is noted for p63 and high molecular weight cytokeratin, markers typically negative in prostatic carcinoma.61 Studies have demonstrated TTF-1 expression in over 50% of small cell carcinomas of the prostate, limiting its use in distinguishing primary small cell carcinoma of the prostate from a metastasis from the lung.59,61,63,64
Because of the rarity of primary small cell carcinoma of the prostate, an important diagnostic consideration is exclusion of metastasis or local extension from other site such as bladder. A technique that can distinguish small cell carcinoma of the prostate from other small cell carcinomas is documentation by fluorescence in situ hybridization (FISH) or reverse transcriptase polymerase chain reaction (RT-PCR) of a gene fusion between members of the ETS family of genes, in particular ERG (ETS-related gene) and TMPRSS2, found in approximately one-half of usual prostatic adenocarcinoma.65 In a similar percent of cases, small cell carcinoma of the prostate is positive for TMPRSS2-ERG gene fusion by FISH.66–71 Importantly, it should be noted that compared to usual acinar carcinoma harboring TMPRSS2-ERG rearrangements, small cell carcinoma with TMPRSS2-ERG rearrangement is not reliably positive for ERG protein by IHC, presumably due to lack of AR expression in small cell carcinoma.66 Additionally, in the setting of standard treatment for CRPC, ERG protein expression may not be present by IHC requiring the use of FISH. According to one study, there is strong and diffuse membrane staining for CD44 in all prostatic NE small cell carcinomas, whereas in usual prostatic adenocarcinomas, only rare positive scattered tumor cells are CD44 positive.43 However, current work by one of the authors have not substantiated this finding and have concluded that this antibody is not useful in the distinction of high-grade adenocarcinoma of the prostate from small cell carcinoma.
The median cancer-specific survival of patients with small cell carcinoma of the prostate in 191 men according to the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2004 was 19 months. Metastatic disease was presented by 60.5% of men, with a decreased survival related to stage. Two- and 5-year survival rates were 27.5% and 14.3%, respectively.72 Given the high rate of occult metastases, clinically localized small cell prostate cancer is typically treated aggressively, often with multimodality therapy with chemotherapy and radiation similar to limited stage small cell lung cancer. Metastatic small cell carcinoma of the prostate is treated with platinum-based combination chemotherapy with regimens similar to those used to treat small cell lung carcinoma.73–76 Some experts treat pure small cell carcinoma with chemotherapy alone, whereas others add ADT.
In summary, small cell carcinoma of the prostate is an aggressive malignancy recognized by relatively typical morphologic features although cases occurring in the prostate may exhibit a slightly wider spectrum of cytologic features than would be allowable at other tumor sites. In tumors showing classic morphology, IHC may not be necessary, although may be frequently useful for confirmation of the diagnosis in view of its important prognostic and therapeutic ramifications.
LARGE CELL NEUROENDOCRINE CARCINOMA
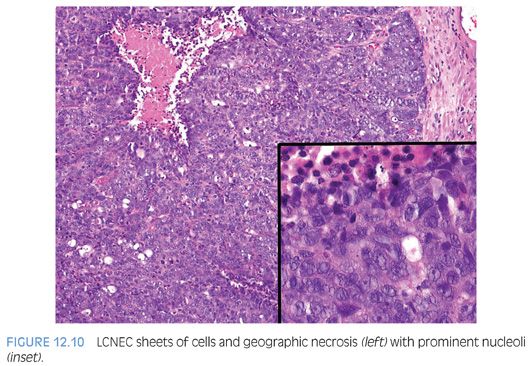
Large cell neuroendocrine carcinoma (LCNEC) of prostate is exceptionally rare, particularly its pure form. The largest series by Evans et al.77 describes seven cases of LCNEC, only one pure and apparently de novo. Six other cases represented progression from prior typical prostate adenocarcinoma, following long-standing hormonal therapy. According to the authors, the large cell NE component was composed of sheets and ribbons of amphophilic cells with large nuclei, coarse chromatin, and prominent nucleoli (Fig. 12.10). Mitotic activity was high, and foci of necrosis were present. The LCNEC component was strongly positive for CD56, CD57, chromogranin A, synaptophysin, and P504S (Fig. 12.11). Ki67 proliferative index was greater than 50%. LCNEC has also been described in association with small cell carcinoma and adenocarcinoma.78
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


