Neurobiology of Psychotic Illnesses
Ann Futterman Collier
Key Questions
• What are the “positive” and “negative” symptoms of schizophrenia?
• What genetic, gestational, and neurologic risk factors are related to schizophrenia?
• How are the dopamine D1 and D2 receptors related to positive and negative symptoms of schizophrenia?
• How is schizophrenia managed?
• What are the hallmark symptoms of major depression?
• What is the neurobiology of major depression?
• What are the subtypes of bipolar disorder?
![]()
http://evolve.elsevier.com/Copstead/
Abnormal behavior is typically considered the opposite of mental well-being. Current internationally recognized clinical diagnostic schemes rely on categorical systems to define mental disorders and provide standardized criteria for each diagnosis. The two most widely established systems are: (1) ICD-10, Chapter V: Mental and Behavioural Disorders, which is part of the International Classification of Diseases published by the World Health Organization,1 and (2) the Diagnostic and Statistical Manual of Mental Disorders (DSM),2 published by the American Psychiatric Association. Although historically there have been significant differences between these systems, in recent years there has been convergence so that both sets of codes are broadly comparable. There are more than 300 different psychiatric disorders listed in the fourth text revision of DSM (DSM-IV-TR), with more added each revision. The fifth edition of DSM (DSM-5) will be published in May 2013.3 Although there will be changes in nomenclature and disorder criteria in the newest version, DSM-5 will continue its clinical utility, be guided more heavily by research evidence, and attempt to maintain continuity with previous editions.
The National Survey on Drug Use and Health (NSDUH) defines serious mental illness (SMI) as a mental, behavioral, or emotional disorder (excluding developmental and substance use disorders) that is diagnosable currently or within the past year, is of sufficient duration to meet diagnostic criteria specified within DSM-IV-TR, and results in serious functional impairment that substantially interferes with or limits one or more major life activities.4 Using the SMI nomenclature, we now know that currently in the United States approximately 5% of all adults meet criteria for SMI. It is also known that 26.2% of the U.S. adult population will have experienced an SMI in the previous year, and 22.3% of these cases will be classified as “severe.” In addition, 46.3% of adolescents between 13 and 18 years of age will be diagnosed with an SMI. Research also shows that SMIs occur more frequently in women than in men, are more prevalent in individuals between the ages of 18 to 49, and are least likely to be seen in American individuals of Asian descent. Table 48-1 provides a review of the most common SMIs by lifetime prevalence; 12-month health care service utilization is also provided for each disorder. Some SMIs have very high lifetime prevalence but only moderate treatment service use (e.g., social phobia). Alternately, other SMIs have low lifetime prevalence but very high treatment service utilization (e.g., schizophrenia). Based on a combination of the lifetime prevalence rates and health care service utilization, the most frequently occurring SMI disorders and those with the highest health care utilization will be reviewed in Chapters 48 and 49.
TABLE 48-1
LIFETIME PREVALENCE OF SERIOUS MENTAL ILLNESS (IN U.S.)
| NAME OF DISORDER | OVERALL CLASSIFICATION | LIFETIME PREVALENCE (%) | AVERAGE AGE OF ONSET (yr) | TREATMENT AND SERVICES USE (ANY TYPE) (%) |
| Social phobia | Anxiety disorders | 29.9% | 13 | 45.6% |
| Major depression | Mood disorder | 16.5% | 32 | 56.8% |
| Attention deficit disorder with hyperactivity (ADHD) | Attention deficit disorder (13-18 yr old) | 9.8% | Not reported | Not reported |
| Posttraumatic stress disorder (PTSD) | Anxiety disorders | 6.8% | 23 | 57.4% |
| Generalized anxiety disorder | Anxiety disorders | 5.7% | 31 | 52.3% |
| Avoidant personality disorder | Personality disorders | 5.2% | Not reported | Not reported |
| Panic disorder | Anxiety disorders | 4.7% | 24 | 65.4% |
| Bipolar disorder | Mood disorders | 3.9% | 25 | 55.5% |
| Obsessive-compulsive disorder | Anxiety disorders | 1.6% | 19 | Not reported |
| Borderline personality disorder | Personality disorders | 1.6% | Not reported | 42.4% |
| Schizophrenia | Schizophrenia | 1.1% | Not reported | 64.3% |
| Antisocial personality disorder | Personality disorders | 1.0% | Not reported | 46.1% |
| Autism spectrum disorders | Autism spectrum disorders | 0.9% | 8 | Not reported |
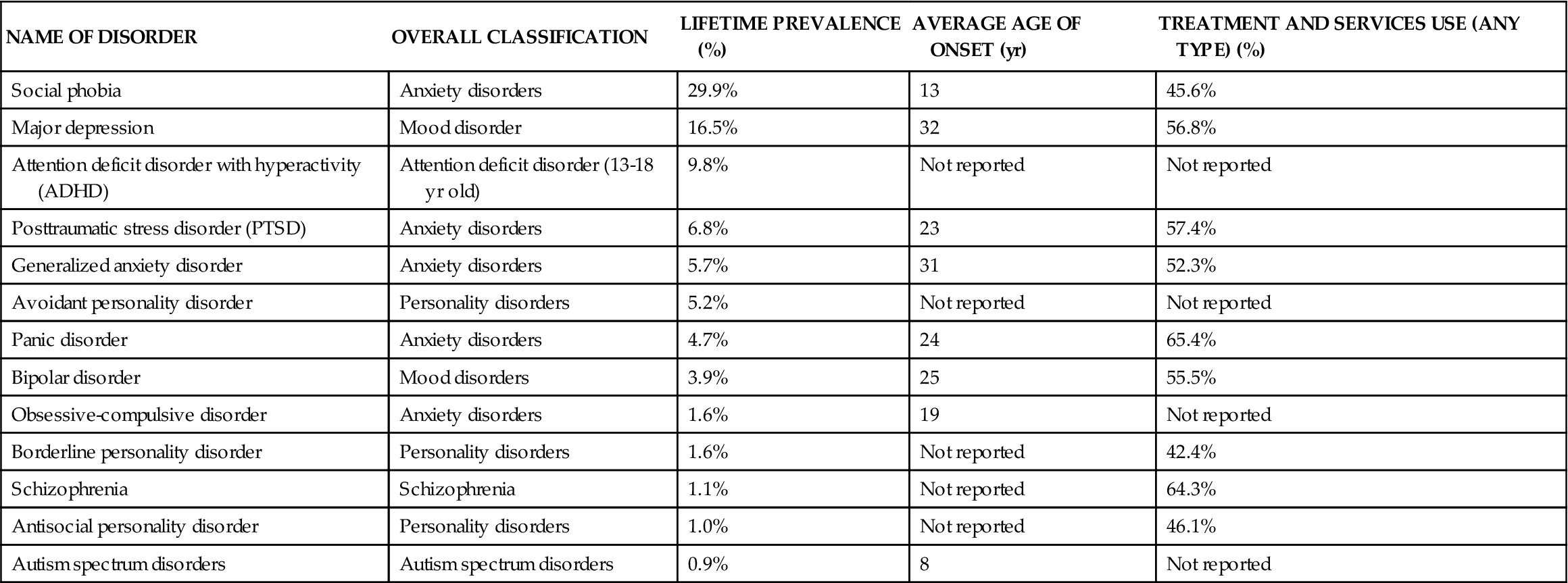
From National Institutes of Mental Health (NIMH). Available at www.nimh.nih.gov/statistics/index.shtml. Accessed 4/25/12.
Thought Disorder, Delirium, and Dementia
Psychosis is a term used to describe a serious and debilitating mental state. The most restricted definition refers to delusions and prominent hallucinations, which occur in the absence of insight into their pathologic basis.2 The broadest definition of psychosis includes other positive symptoms that are characteristics of schizophrenia, such as disorganized speech and grossly disorganized or catatonic behavior. Hallmark symptoms of psychosis are delusions, hallucinations, cognitive disorganization, and altered reality. These symptoms characterize a small number of specific mental disorders; however, a wide range of different physical and mental conditions can produce psychotic symptoms. The neurobiological basis of psychosis can be summarized as acute or chronic alterations in neuron anatomy and physiology and cellular biochemical processes. Although psychosis occurs most often with schizophrenia and mood disorders, it may also manifest with substance disorders, delirium, dementia, amnestic disorders, and acute stress disorder. Groundbreaking research in brain imaging techniques and psychopharmacology now allows highly precise definitions of the various biochemical pathways associated with psychosis. This chapter addresses three disorders that are associated with psychosis: schizophrenia, major depressive disorder (MDD), and bipolar disorder (BD).
Schizophrenia
Schizophrenia refers to a chronic, remitting, and relapsing psychotic disorder that is associated with significant impairment in social and vocational functioning.5,6 Literally, the term is defined as “split mind,” and once was believed to be a disorder that caused the personality to split into multiple subtypes. Schizophrenia is now correctly understood as a split or separation among normally well-synchronized brain functions. This loss of synchronized brain functioning leads to thoughts, behaviors, and feelings that are disordered, disorganized, and disconnected from reality—a condition generally referred to as psychosis.7
The global incidence rate of schizophrenia has consistently been estimated to be about 1% of the world population,7 and is fairly equally distributed across genders. Unfortunately, schizophrenia appears to be associated with an average lifespan reduction of 15 to 25 years.5,6,8 Women show symptom onset and are diagnosed typically between the ages of 25 and 35 years, men between the ages of 15 and 25 years. Women with schizophrenia appear to have better outcomes than men.9 Possibly, the later age of onset, the protective nature of female hormones such as estrogens, or a better drug response accounts for this difference.9 People with schizophrenia may be at increased risk for type 2 diabetes and cardiovascular disorders,10–13 perhaps attributable to the side effects of antipsychotic medications, poorer overall physical health, less healthy lifestyles, and substandard health care. However, newer antipsychotic medications alone do not appear to account for the increased incidence of diabetes and cardiovascular disease.10–12
Etiology and neurobiology
Dopamine effects
Several decades ago it was hypothesized that abnormalities in dopaminergic pathways in specific regions of the brain were the cause of schizophrenia. This conclusion was reached after noting that dopamine antagonists (competitive) reduce symptoms of schizophrenia whereas dopamine agonists (complementary) produce schizophrenic symptoms. Dopamine-specific neurons in the brain primarily are located in the ventral tegmentum of the mesencephalon, medial and superior to the substantia nigra. These regions, as a whole, are referred to as the mesolimbic dopaminergic system. The long nerve fibers leaving this system mainly project into the medial and anterior portions of the limbic system. The limbic system contains three powerful centers of behavior control: the nucleus accumbens, the amygdala, and the anterior caudate nucleus.
Generally speaking, we now know that decreased neurotransmission and connectivity is the neurobiological basis of schizophrenia. Frankle and colleagues14 reported that the core biochemical process of schizophrenia involved an excess of subcortical dopaminergic transmission at dopamine D2 receptors and a deficit of glutamate transmission at N-methyl-D-aspartate (NMDA) receptors. The dopamine pathogenesis of schizophrenia can be thought of as disordered synaptic organization. In the brain, normal synaptic organization implies the provision for normal communication among brain cells. Specific schizophrenia symptoms have been associated with neurotransmission dysregulation that diminishes or elevates dopamine activity. Hyperdopaminergic states have primarily been associated with positive symptoms of schizophrenia, such as hallucinations, delusions, and psychosis. Hypodopaminergic states have primarily been associated with negative symptoms of schizophrenia, such as cognitive difficulties, lack of energy and motivation, and depression.15 Dopamine D2 receptors have been associated with positive symptoms and psychosis, whereas dopamine D1 receptors have been associated with negative symptoms.
That said, dopamine receptor activity and synaptic transmission are subject to a variety of mediators such as brain-derived neurotrophic factor (BDNF), a neurotrophin that increases synaptic activity and neurotransmitter output.16 Dopamine synaptic activity also has been closely linked with stress-related cortisol activity and drugs of abuse (cocaine, amphetamines, morphine, nicotine, and ethanol).17 Although dopamine dysregulation clearly is the driving force behind the neurochemical processes of psychosis, other experts suggest considering the role of biopsychosocial contextual factors in shaping and aggravating the expression of biochemical dysregulation.18
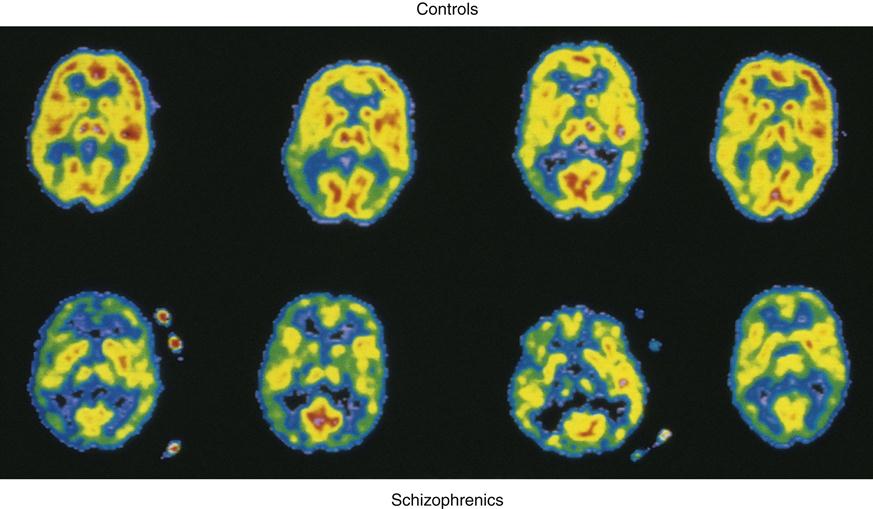
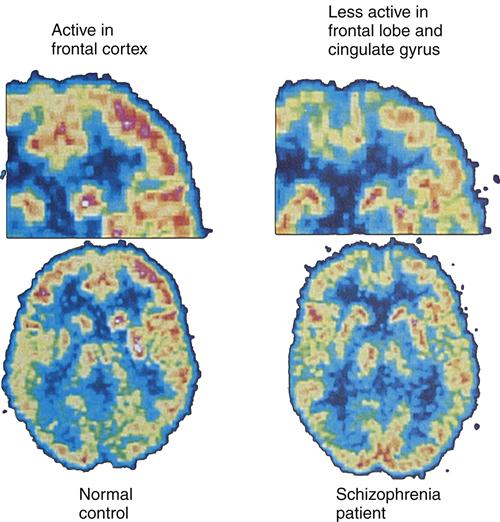
Positron emission tomography (PET) brain images have enabled researchers to further examine the overall role of dopamine activity in psychosis and schizophrenia. PET studies have demonstrated low glucose metabolism rates in the frontal cortex and dopamine regions19 of the brains of persons with schizophrenia (Figure 48-1). Of the many different types of dopamine brain cell receptors identified, dopamine D2 receptors once again were found to be strongly associated with symptoms of schizophrenia. Similar research has shown that dopamine D2 receptors are particularly responsive to antipsychotic drugs.
Thus, the dopamine hypothesis, which postulates that schizophrenia symptoms result from presynaptic dysregulation of dopamine transmission, continues to be the focus of neurobiological studies. The specific neuropathologic mechanism of schizophrenia appears to be caused by a functional excess of postsynaptic dopamine receptor activity and dopamine receptor hypersensitivity, either alone or in combination.
Genetic effects
In the absence of any other condition, a family history of schizophrenia by itself does not lead to schizophrenia: Schizophrenia can and does develop in persons with no family history of the disease. Initial interest in identifying possible genetic contributions to schizophrenia was based on early research on monozygotic (identical) and dizygotic twins, by studying twins born to parents with schizophrenia but reared apart from their parents. Results suggested that nearly 50% of these offspring developed schizophrenia.20 Children of two parents with schizophrenia have a 40% to 68% risk of developing the illness, whereas children with one parent with schizophrenia have a 9% to 16% risk.9 The risk of a non–twin sibling of a brother or sister with schizophrenia developing the disease is slightly less at 8% to 14%. The obvious limitation of these findings is that they do not explain why an at-risk offspring does not develop schizophrenia.
Several additional conditions are thought to be involved in the transformation from genetic risk to actual illness. Factors such as prenatal infections, malnutrition, birth complications, and brain injury have been associated with the development of schizophrenia in persons who have increased genetic risk.21 Moreover, different gene locations are also relevant to the illness onset. Experts question whether inherited genetic risk for schizophrenia could explain observed differences in incidence and prevalence rates based on gender and race. Lastly, genetic models of schizophrenia typically do not address risk in terms of the subtype of schizophrenia, such as paranoid schizophrenia as opposed to schizophrenia without paranoia. Given the high standards of proof required for gene typing, a purely genetic explanation of an illness as complex as schizophrenia seems unlikely. Increased genetic risk of schizophrenia appears to be a critical part of a puzzle composed of many pieces. Thus, for individuals who are genetically predisposed, some aspects of schizophrenia will probably be determined by biopsychosocial characteristics.22 Researchers continue to study the actual versus potential genetic risks of schizophrenia.
Gestational effects
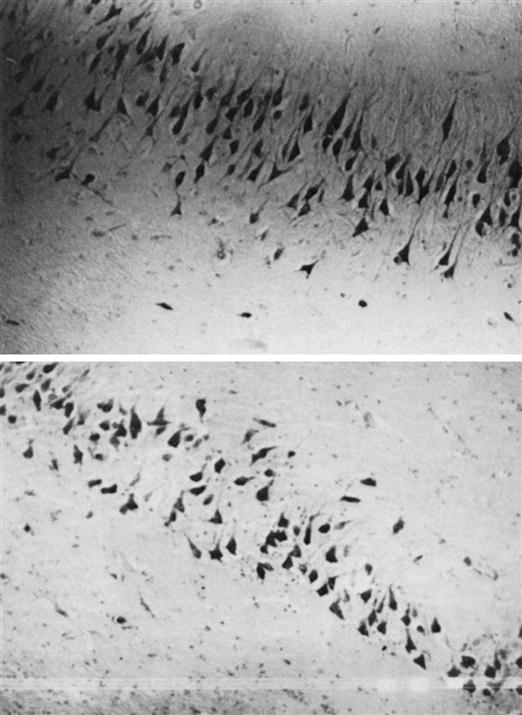
Early findings noted that in persons with schizophrenia, pyramidal cells in the hippocampus were not lined up like a “picket fence,” as they were in control subjects (Figure 48-2). Instead, the cells appeared to be rotated at 70-degree to 90-degree angles.23 Pyramidal cells migrate during the second trimester of gestation and later become fastened by neuronal cell adhesion molecules (N-CAMs). Researchers focused on the possibility of pyramidal cell misalignment and lost N-CAM adhesive effects as links between gestation and schizophrenia. Researchers noted that in pregnant women living in Scandinavia and England who were exposed to the 1957 flu epidemic during their second trimester, 300% more of their children were diagnosed with schizophrenia than those of women who experienced flu during the first or third trimester.
These findings were considered significant for two reasons: (1) neuronal migration peaks during the second trimester, and (2) the influenza virus is one of very few viruses that produce capsular neuraminidase, an enzyme that can change the adhesive properties of N-CAMs.24 Hippocampal, parahippocampal gyrus, and amygdala neurons process information and emotional expression. Other researchers have suggested that delivery complications could be an additional factor, playing a mediating role between prenatal influenza exposure and later development of schizophrenia.25
More recent studies of gestational abnormalities focus on specific brain regions and stages of prenatal neurodevelopment.26 This research is exemplified in a well-controlled Canadian study that examined the association of hippocampal formation abnormalities and first adult episode of schizophrenia. The main functions of the hippocampus are learning and new-memory formation. Both abilities may be lost when hippocampal functioning is impaired. Macroscopic cell abnormalities, such as fewer synapse connections and diminished synapse activity, result in reduced hippocampal volume. Reduced hippocampal volume has been associated with severe stress, mood disorders, and schizophrenia.27 The Canadian researchers hypothesized that schizophrenia was associated with incomplete formation of the hippocampus during the second trimester of development. They used magnetic resonance images to compare the brains of newly diagnosed patients with healthy matched controls. Although the number of participants in the study was too small to allow for generalizations to be drawn, the magnetic resonance images of the newly diagnosed patients clearly showed enlarged hippocampal fissures or disrupted hippocampal formation. Interestingly, obstetric complications during pregnancy were not significant factors.
Neurologic effects
Observed neuroanatomic differences in persons with schizophrenia led researchers to study anatomic and functional abnormalities in the limbic region and frontal lobe parts of the brain. Structural abnormalities in these brain regions would suggest that abnormal functioning might contribute to the disrupted cognitive processes or symptoms of schizophrenia. Beginning in 1976, computed tomography (CT) studies revealed enlarged brain ventricles in persons with schizophrenia. A groundbreaking 1980 study showed that the neurochemical basis of schizophrenia might involve two processes: dopamine neurotransmission dysregulation and abnormal cerebral structure.28
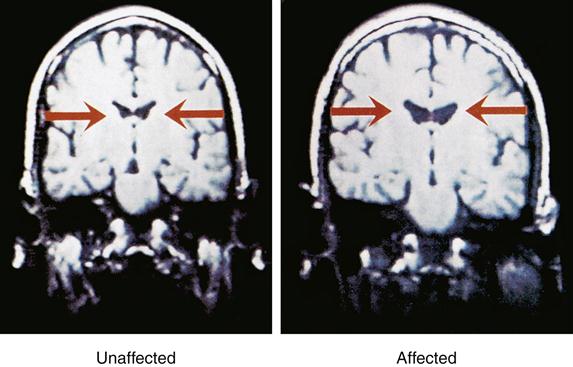
Magnetic resonance imaging (MRI) studies of persons with chronic schizophrenia indicated larger-than-normal lateral and third ventricles and reduced temporal lobe gray matter29 (Figure 48-3). MRI findings also showed reduced frontal lobe blood flow and relative decreases in frontal lobe metabolic activity. Prefrontal cortex structure and functioning deficits were consistently observed when the subject was simultaneously placed under stress. The stress used in the study was primarily psychological, such as contingency planning exercises or divergent thinking during performance of a cognitive task that utilized specific regions of the prefrontal cortex.30
Subsequent studies of brain structure in persons with severe schizophrenia replicated the earlier findings of abnormal limbic-cortical structures and smaller, misarranged hippocampal pyramidal cells. This includes replication of MRI findings of reduced bilateral temporal lobe volume, decreased hippocampal volume, and reduced volume in the parahippocampal gyrus region of the brain. Studies of brain regions other than the limbic system revealed frontal lobe structural alterations in the dorsolateral area of the prefrontal cortex and in the cingulate and motor cortices. The finding of decreased frontal lobe glucose metabolism associated with schizophrenia also has been replicated (Figure 48-4).31
Postmortem brain tissue studies indicated fewer nicotinic receptors present in the hippocampus of schizophrenics. Tobacco dependence is a common secondary disorder with schizophrenia. The finding of fewer nicotinic receptors was of particular interest to researchers in that previous studies had shown that smoking could temporarily normalize auditory sensory gating that typically becomes impaired with schizophrenia.32 Since then, researchers found that a neurophysiologic deficit at chromosome 15, at the OC7 nicotinic receptor gene, may partially explain the inheritance of this neurophysiologic symptom.33 Although the development of schizophrenia is associated with any number of specific abnormalities, early neurodevelopmental alterations that result in dysfunction of the limbic and prefrontal regions of the brain appear to be critical.
Clinical manifestations
Schizophrenia is characterized by positive (reality distortion and disorganization), negative, cognitive, and mood symptoms.7 The types and severity of symptoms differ among patients and change over the course of the illness. Positive symptoms typically reflect an excess or distortion of normal functions. In contrast, negative symptoms reflect a decrease or loss of normal functions. Positive symptoms include the psychotic dimension, or distortions in thought content (delusions) and perception (hallucinations), as well as the disorganization dimension, or disorganization in speech and behavior. The resulting positive symptoms also include the inability to self-monitor behavior, which results in grossly disorganized or catatonic behavior. Delusions, or systematic, fixed, false beliefs, usually involve themes of persecution, reference, somatization, religiosity, or grandiosity. Hallucinations are sensory perceptions with no apparent stimulus. They occur in any sensory system (such as auditory, visual, olfactory, gustatory, or tactile) but usually auditory hallucinations are the norm. Auditory hallucinations are commonly experienced as voices, distinct from the person’s own thoughts, and out of the range of normal experience. Disorganized thinking is usually evaluated by an individual’s speech, and is frequently characterized by frequent derailment or loose associations, invented words, tangential ideas, and, when most severe, incomprehensible speech. Grossly disorganized behavior can range from childlike silliness to unpredictable agitation, and impairs the individual’s ability to complete tasks of daily living. When individuals display catatonic motor behaviors, they show a decrease in reactivity to environmental events, to such an extreme that they can maintain a rigid posture and resist efforts to be moved. The positive symptoms13 of schizophrenia are thought to result from excessive dopamine D2 receptor activity in the brain. Negative symptoms are considered to be restricted affect, or avolition and asociality. Negative symptoms represent deficits in functioning and can be more difficult to recognize than positive symptoms. Negative symptoms13 of schizophrenia are considered to be associated with dopamine D1 receptor activity in the brain. Poor cognitive functioning in schizophrenia includes difficulties with memory, attention (e.g., poor concentration, distractibility, selective attention), and decision making.
According to the workgroup responsible for developing DSM-5,3 the active phase of schizophrenia is characterized by two or more of the following symptoms that must be present for a significant portion of time during a 1-month period (or less if successfully treated): (1) delusions; (2) hallucinations; (3) disorganized speech; (4) grossly abnormal psychomotor behavior; and (5) negative symptoms. At least one of these symptoms should include delusions, hallucinations, or disorganized speech. In addition, for a significant portion of the time since the onset of the disturbance, schizophrenia is also characterized by social/occupational dysfunction in one or more major areas: work, interpersonal relations, or self-care. All of these are markedly below the level achieved before the onset of the illness (or when the onset is in childhood or adolescence, failure to achieve expected level of interpersonal, academic, or occupational achievement). Regarding duration, typically continuous signs of the disturbance exist for at least 6 months, with at least 1 month of symptoms (or less if successfully treated) that meet criteria for the active phase (delusions, hallucinations, disorganized speech), and may include periods of prodromal or residual symptoms. During these prodromal or residual periods, the signs of the disturbance may be manifested by only negative symptoms or by two or more of the active-phase symptoms in an attenuated form (e.g., odd beliefs, unusual perceptual experiences). Finally, for an individual to receive the diagnosis of schizophrenia, the diagnoses of schizoaffective disorder and mood disorder with psychotic features must be ruled out. In addition, symptoms cannot be due to the direct physiologic effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition.7
Schizophrenia has been divided into five subtypes in DSM-IV-TR:2 paranoid; disorganized; catatonic; undifferentiated; and residual. In each subtype there is predominant symptomatology at the time of evaluation; these can change over time, such as at a subsequent diagnosis. In addition, an individual may have symptoms characteristic of more than one subtype. DSM-V may introduce a different classification of subtypes. The paranoid and disorganized types are the least severe (in that order). The undifferentiated type is essentially a catch-all category for individuals who do not meet criteria for any of the other subtypes. The residual type indicates that the disturbance has continued, but the active-phase symptoms are no longer met.
Pharmacologic treatment
When untreated, schizophrenia is associated with increased mortality, impaired vocational and social functioning, and reduced quality of life.5 Unfortunately, the extent to which treatment does actually improve life span and psychosocial functioning is not specifically clear. Because antipsychotic medications do decrease the likelihood of relapse, they are encouraged.
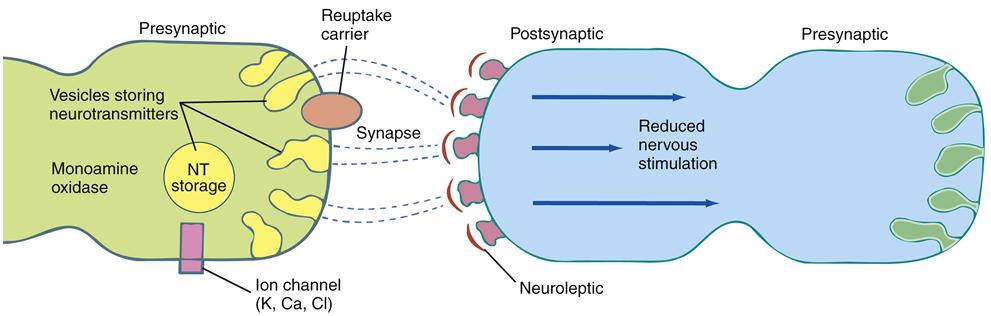
When effective, antipsychotic medication can be expected to diminish or remit hallucinations or reduce their impact on functioning.34 The ability to reason should improve; ambivalence, delusions, and suspiciousness should be greatly reduced; agitation and confusion should be relieved; and social behavior should improve. Antipsychotic medications are designed to have specific effects on targeted neurotransmitters. Newer antipsychotic medications, typically called second-generation agents (SGAs) (atypical), are intended to generally manage psychosis as well as both positive and negative schizophrenia symptoms.5 Older antipsychotic medications, traditionally called first-generation agents (FGAs) (conventional), were less effective in managing negative schizophrenia symptoms. They mainly acted as dopamine antagonists, blocking the dopamine receptors (D2). This action diminishes the amount of dopamine received by the receptor sites (Figure 48-5).
All FGAs and SGAs appear to be equally effective in reducing the positive symptoms and disorganization associated with schizophrenia, hence blocking the dopamine D2 receptor.5,34 Neither antipsychotic agent appears to improve the cognitive symptoms in patients with schizophrenia; in fact, findings suggest that they worsen cognitive impairment.5 Although the maximum drug response may not be reached for many months, patient response over the first 2 to 4 weeks of antipsychotic medication use will typically predict long-term response. Antipsychotic response will vary as a result of the stage of illness, with first-episode patients responding faster and at a higher rate than those at later stages.5 In addition, both FGAs and SGAs play a substantial role in decreasing the likelihood of relapse.
FGAs and SGAs differ most notably in their side effect profiles and their potential for interacting with other medications. FGAs (e.g., chlorpromazine) had relatively nonspecific neurotransmitter effects and numerous side effects, the most difficult being extrapyramidal symptoms (EPS). Haloperidol was the first FGA that had significantly less anticholinergic and hypotensive side effects. By 1990, SGAs became widely available; the SGAs have generally shown a lower risk of EPS but a higher risk of other metabolic adverse effects. The first SGA, clozapine, was quickly followed by a generation of new atypical antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone), each promising still fewer side effects, better relief of both positive and negative schizophrenic symptoms, and minimal risk of EPS, specifically those associated with tardive dyskinesia. Some worrisome side effects are clinically significant weight gain, glucose dysregulation, and dyslipidemia.35,36 Experts have speculated that hyperglycemia may have to do with dopamine receptor involvement in the regulation of insulin secretion. Numerous explanations for weight gain as a major side effect of antipsychotic medication continue to be developed and tested. The glutamate neurobiological model37 of psychosis and schizophrenia, an alternative to the dopamine model, may be the basis for the next new generation of antipsychotic medications.
The glutamate deficit37 model attempts to focus attention on the cause of excessive dopamine receptor activity rather than the excessive activity itself. γ-Aminobutyric acid (GABA) is the most important inhibitory brain neurotransmitter. GABA synthesis depends on and is controlled by the enzyme glutamic acid decarboxylase (GAD). GAD activity is modulated by the glutamate receptor NMDA. GAD dysregulation is thought to lead to insufficient GABA activity and, consequently, excessive dopamine activity. Reduced GAD activity has been observed in the dorsolateral prefrontal cortex and hippocampus of patients with schizophrenia. The interesting observation for pharmacologic researchers is that a broad range of different drugs has been shown to be capable of affecting GAD activity. Although the glutamate deficit model is not new, the model still may lead to a class of antipsychotic medications unlike any previous generation.
Dopamine D2 continues to remain the lead neurotransmitter target of the atypical antipsychotics.34 Researchers found that the most common side effects of antipsychotic medication could be reduced if the drug’s impact on dopamine D2 receptors was not excessive. Lower receptor occupancy, less receptor affinity, and faster release38 of the receptor were methods shown to be associated with fewer side effects. More recently, the aim for effective antipsychotic medication is to stabilize rather than reduce dopamine activity. The newest SGAs, such as aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone, show greater affinity for serotonin receptors (negative symptoms) and moderate affinity for dopamine and norepinephrine receptors (positive symptoms).5,38 Effective antipsychotic medications have significant dopamine effects; however, schizophrenia symptoms are highly complex and likely to involve other neurotransmitters, particularly serotonin and norepinephrine (Table 48-2).
TABLE 48-2
NEUROTRANSMITTERS IN SCHIZOPHRENIA: TYPE AND FUNCTION
| NEUROTRANSMITTER | TYPE | FUNCTION |
| Dopamine | Catecholamine | Regulates motor behavior in extrapyramidal nerve tracts and also transmits in cortex. Increases vigilance and may increase aggression. Excess may produce psychosis; deficiency may cause movement disorders (EPS). |
| Serotonin | Indolamine | Brainstem transmitter; modulates mood; lowers aggressive tendencies. Deficiency may be responsible for some forms of schizophrenia. |
| Acetylcholine | Cholinergic | Transmits at nerve-muscle connections (central nervous system and autonomic nervous system). Deficiency may increase confusion and acting-out behavior. Controls EPS. |
| Norepinephrine | Catecholamine | Transmits in sympathetic nervous system. Induces “fight or flight” syndrome (hypervigilance). May be insufficient in clients with schizophrenia who display anhedonia (loss of pleasure). |
| Cholecystokinin | Peptide | Excites limbic neurons. Deficiency is related to avolition (lack of motivation) and flat affect. |
| Glutamate | Amino acid | Excitatory neurotransmitter. Impairment in N-methyl-D-aspartate affects glutamate metabolism, which can lead to problems with cognition, delusions, and possibly some negative symptoms of schizophrenia. |
| γ-Aminobutyric acid (GABA) | Amino acid | Inhibitory neurotransmitter; predominantly a brain transmitter. Promotes balance between dopamine and glutamate and thus inhibits impulsive behaviors. |
From Fortinash KM, Worret PA: Psychiatric mental health nursing, ed 5, St Louis, 2012, Elsevier, p 265.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree