Alterations in Special Sensory Function
Joni D. Marsh
Key Questions
• What are the general manifestations of hearing impairment?
• How do conductive and sensorineural mechanisms of hearing loss differ in etiology and treatment?
• What are the predisposing factors, clinical manifestations, and management of otitis media?
• What are the general manifestations of visual impairment?
• How do open-angle and acute angle-closure glaucoma differ?
![]()
http://evolve.elsevier.com/Copstead/
The human body has countless sense organs that fall into two main categories: general sense organs and special sense organs. By far the most numerous are the general sense organs or receptors. The receptors function to produce the general or somatic senses. Examples of these senses are touch, temperature, and pain, and the receptors that initiate various reflexes necessary for maintaining homeostasis (see Chapter 43). The largest general sense organ in the body is the skin.
Special sense organs, by comparison, function to produce the unique sensations of hearing, balance, vision, smell, and taste. These senses allow humans to interact with their environment in a meaningful way.
Alterations in sensory function may be acute/short-term, chronic/long-term, or progressive in nature. They may result from such factors as genetics, disease, infection, trauma, and normal aging. Alterations in special sensory function require prompt assessment, evaluation, and treatment from appropriate health professionals. Equally important is an assessment of how the sensory impairment affects the individual’s activities of daily living. This chapter discusses special sensory function with regard to physiologic processes, sensory impairment, and the diagnosis and management of these impairments.
Hearing and Balance
Structure and Function of the Ear
External Ear
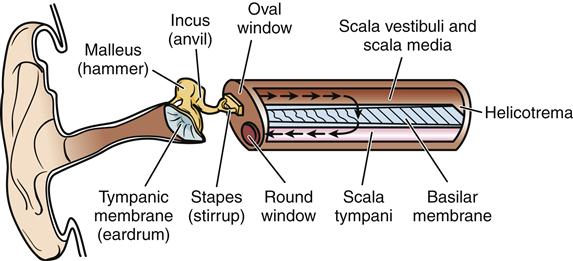
Hearing results from normal functioning of several complex structures both external and internal to the body. Sound consists of waves of vibrations in the air, produced in the environment. These vibrations travel much like ripples in a pool of water. Externally, these vibrations are caught and funneled into the ear canal by the auricles (Figure 46-1). Even though the auricles are in a fixed position and lie close to the head, their shape serves to concentrate sound waves, especially high-frequency waves. The auricles also have an important role in sound localization.
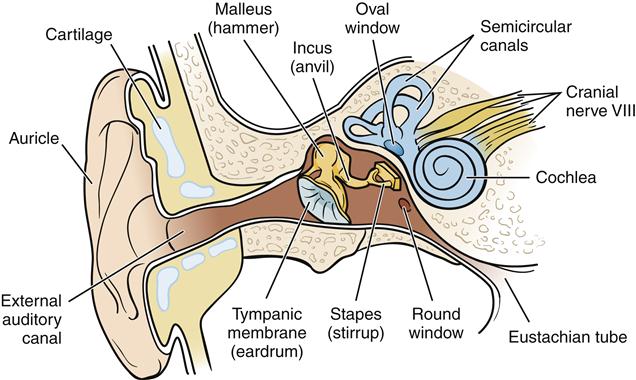
The ear canal has a somewhat S shape from its opening to its termination at the tympanic membrane. This configuration affords both protection from airborne foreign objects and access to sound. The outer portion of the ear canal contains hair to filter out unwanted substances. Along the ear canal are also glands that secrete cerumen. This brown, waxlike substance coats the hairs in the canal to help prevent the entrance of foreign bodies into the ear canal. After entering the ear canal, sound waves strike the tympanic membrane (eardrum) and cause it to vibrate. The tympanic membrane is a thin, elastic membrane that is very sensitive to changes in pressure.
Middle Ear
The middle ear is a bony, air-containing space that functions primarily as a structure by which sound energy is transmitted from the air to the fluids of the inner ear. The tympanic membrane is connected to the first of the ossicles, the malleus (hammer), followed by the incus (anvil) and stapes (stirrup). The ossicles further amplify the sound waves and then transfer airborne sound waves to the fluid-filled inner ear at the oval window.
The eustachian tube is also part of the middle ear, and although it does not contribute directly to the transmission of sound through the ear, absence of proper function can greatly affect hearing. This tube has a mucosal lining and extends from the middle ear cavity to the nasopharynx. It makes equalization of pressure against the inner and outer surfaces of the tympanic membrane possible, thus improving mobility of the membrane for sound transduction. A patent eustachian tube prevents membrane rupture and discomfort that marked pressure differences can produce.
Inner Ear
The inner ear is composed of the oval window, the cochlea, and the semicircular canals. Within the cochlea are three parallel tubes: the scala vestibuli, the scala media, and the scala tympani. Movement of the perilymph, a fluid much like cerebrospinal fluid, in the scala vestibuli and the scala tympani is eventually dissipated by movement of the round window (Figure 46-2). The scala tympani and the scala vestibuli are continuous with one another at the apex of the cochlea through an opening called the helicotrema.
Transmission of the sound stimulus from the scala vestibuli to the vestibular membrane results in displacement of the endolymph, the fluid contained in the membranous labyrinth of the scala media and the basilar membrane. The organ of Corti, which contains the receptors for hearing, lies on the basilar membrane. Perilymph and endolymph transmit the mechanical vibrations from the footplate of the stapes to the organ of Corti. Endolymph also transports nutrients to the organ of Corti. No direct communication between endolymph and perilymph is normally present.
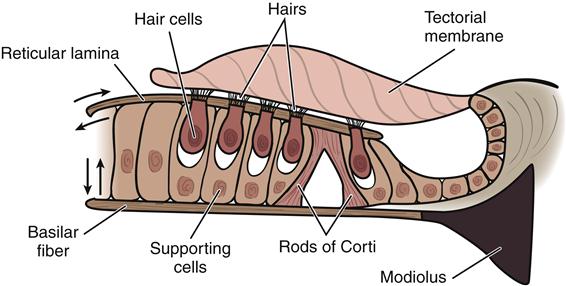
The organ of Corti consists of a series of sensory hair cells and supporting cells. These cells are innervated by the sensory fibers from the vestibulocochlear nerve (cranial nerve VIII). Overhanging the organ of Corti is a flexible flap of tissue called the tectorial membrane (Figure 46-3). Hairs of the sensory cells of the organs of Corti are in contact with the tectorial membrane. The wave of perilymph induces movement of the basilar membrane, which causes a pull or shearing force on the hair cells in contact with the tectorial membrane. This action transforms the mechanical energy of sound into electrical impulses stimulating the vestibulocochlear nerve. Impulses are relayed through nuclei in the medulla, pons, midbrain, and thalamus before reaching the auditory area of the temporal lobe.
Balance
The ear has dual sensory functions. In addition to its role in hearing, it also functions as the sense organ of equilibrium. The stimulation or “trigger” responsible for balance involves activation of receptor hair cells contained in the semicircular canals. Movement of the head causes movement of the endolymph contained in the semicircular canals. The receptor hair cells in turn create a nerve impulse in the vestibular portion of the vestibulocochlear nerve (cranial nerve VIII), where the stimulus is transmitted to the brain. Signals from the inner ear are involved not only in keeping individuals upright but also in controlling the eye muscles so that the eyes can remain fixed on the same point despite changes in the position of the head.
Vertigo
Vertigo is a common symptom of vestibular disorders rather than a well-defined disease. It is either a sensation of motion without any actual motion or an exaggerated sense of motion; it is not simply a sensation of “spinning.” Accompanying symptoms may include nausea, vomiting, pallor, and sweating. Nystagmus often is also noted. It is not associated with a loss of consciousness or a feeling of impending loss of consciousness more common to syncope. Vertigo can have a peripheral vestibular cause or central causes. In the case of Meniere disease (discussed later in this chapter), a disorder in which vertigo is common, the cause is unknown.
Disorders of the brainstem or cerebellum that may also cause vertigo include tissue ischemia secondary to atherosclerosis, tumors, or conditions such as psychiatric disorders, migraine headaches, or multiple sclerosis. However, in these cases, additional neurologic signs and symptoms are typically present. Drugs may also cause vertigo (alcohol, anticonvulsants, sedatives). Management of vertigo is usually aimed at the cause if known. Medications such as antihistamines and anticholinergics can be helpful.1
General Manifestations of Hearing Impairment
Hearing impairment is a very common disorder and a leading chronic health problem in the United States. Approximately 17% (36 million) of American adults report some degree of hearing loss. Out of 1000 U.S. children, 2 to 3 are born deaf or hard of hearing.2
Hearing may be impaired in many ways, and impairments can occur across the age spectrum. Disorders may affect the outer ear, such as impacted cerumen and foreign bodies in the ear canal. The middle ear may be affected by fluid effusion, infection (otitis media), tumors, or diseases such as otosclerosis. Hearing loss may also be caused by repeated exposure to loud sounds or ototoxic medications such as aminoglycoside antibiotics, chemotherapeutic agents, and high-dose loop diuretics. Some of these medications can cause hearing loss even when administered at therapeutic doses.3 Other causes of hearing loss, especially in children, include infection (measles, meningitis), environmental teratogens (radiation), intrauterine infections (cytomegalovirus, herpes simplex virus, human immunodeficiency virus, and Toxoplasma), maternal metabolic disorders (diabetes, hypothyroidism), and exposure to industrial chemicals (solvents or pesticides). In adults poor nutrition and smoking also contribute to hearing loss.4,5 By whatever mechanism hearing impairment occurs, the signs and symptoms are similar.
Symptoms of hearing impairment may be manifested in behavior such as inattentiveness, speaking out of turn in conversations, withdrawal from social situations, increased volume of voice when speaking, increased volume of radio or television, confusion, loss of reaction to loud sounds, and emotional outbursts. Children with hearing impairment may demonstrate inattentiveness and difficulty with articulation and the development of speech. Alterations in hearing function can generally be classified into two categories, conductive and sensorineural, depending on the cause of the impairment. Some hearing impairments have a component of both. The following alterations in hearing function are categorized according to the primary cause of dysfunction.
Hearing Impairment Disorders
Conductive Hearing Impairment
Conductive hearing loss occurs when sound cannot reach the cochlea. Individuals with conductive hearing impairment have a decreased sensitivity to sound. This type of hearing impairment is caused by dysfunction in the external or middle ear. Four mechanisms, each resulting in impairment of the passage of sound vibrations to the inner ear, lead to conductive hearing impairment: (1) obstruction (cerumen impaction), (2) mass loading (middle ear effusion), (3) stiffness effect (otosclerosis), and (4) discontinuity (ossicular disruption). Conductive hearing loss is generally correctable with medical or surgical therapy—or in some cases both.3
Loss Caused by Cerumen Impaction and Foreign Body Occlusion
Etiology
Cerumen impaction is a common and frequently overlooked cause of conductive hearing loss, especially in the elderly. In most cases, cerumen impaction is self-induced through attempts at cleaning the ear with objects such as cotton swabs. Foreign bodies in the ear canal occur most frequently in children. Objects such as small stones, pieces of wood, peas, beans, and paper are fairly common.
Clinical manifestations and treatment
Sometimes no symptoms are present and the foreign body is discovered on routine examination. If the foreign body is an insect, beating of its wings and movement may cause distress. When symptomatic, however, foreign bodies can cause pain or drainage of pus from the ear. The external ear canal is very sensitive to touch and bleeds easily, which increases the risk for subepithelial hematomas from minor trauma. Therefore, removal of solid foreign bodies carries a risk of additional trauma to the ear canal, as well as tympanic membrane rupture, if the individual is not completely cooperative or removal is difficult. Light anesthesia may be necessary. Firm materials may be removed from the canal with loop or hook instruments, taking care not to push the object further into the canal. Irrigation should not be performed on organic foreign bodies (beans, peas) because water may cause them to swell. Living insects may be immobilized with lidocaine before removal.3 Excess cerumen may be removed with gentle irrigation.
Otosclerosis
Etiology
Otosclerosis is a progressive conductive, sensorineural, or mixed hearing impairment caused most often by stapedial fixation. Resorption of bone is followed by the formation of new spongelike bony lesions usually occurring on and around the ossicles of the middle ear. Lesions involving the footplate of the stapes cause decreased transmission of sound waves to the oval window. However, when otosclerotic lesions impinge on the cochlea, permanent sensorineural hearing loss can occur. The basic initiating factors are unknown, but up to 50% of individuals with clinical otosclerosis have a history of the disease in the family.6 Several types of inheritance patterns have been suggested. The disease is most common in Caucasian middle-aged women. Pregnancy may accelerate the otosclerotic process.6 Viral infections and autoimmune processes are also thought to be possible causes. The age of onset is variable due to the insidious progression of the disorder, but the most common ages are between 15 and 45. There may be periods of symptom worsening, followed by times of little apparent change.
Diagnosis and treatment
The diagnosis of otosclerosis is made through careful history taking and radiologic studies, along with audiometric studies. Generally, hearing loss begins in one ear, but 80% to 90% of affected individuals will develop bilateral impairment.6 Although hearing loss may be severe, speech discrimination is preserved except in the instance of cochlear involvement. The individual may report being able to hear better in a noisy environment than in a quiet one. Tinnitus is often present. Hearing tests reveal a conductive loss of varying severity.
Management of otosclerosis at this point is generally surgical, in an effort to prevent the conductive hearing loss. The limitation on treatment options for otosclerosis is related largely to the lack of exact knowledge regarding the cause and pathogenesis of the disease. The universally accepted operation for otosclerosis is stapedectomy, or removal of the focus of the disease by removing the stapes and inserting a prosthesis. In the case of otosclerosis involving the cochlea, treatment with oral sodium fluoride and bisphosphonates is associated with some decrease in development of the sensorineural hearing loss.7 Amplification with hearing aids is another approach.
Sensorineural Hearing Impairment
In sensorineural hearing impairment, the hearing mechanism is disturbed in the inner ear in the cochlea or the vestibulocochlear nerve to the brain. Long-term exposure to loud sounds, ototoxic medication, trauma, metabolic causes, aging, and certain disease states cause sensorineural hearing impairment. Sensorineural hearing loss is usually irreversible. Progress, however, is being made in using novel approaches to protect the hair and supporting cells of the inner ear.8
Loss Caused by Ototoxic Medications
Drug toxicity is an increasingly important cause of sensorineural hearing loss. The drugs most well-known for this effect are the aminoglycoside antibiotics, salicylates, quinine and related antimalarials, and cytotoxic antineoplastic drugs. Most ototoxic drugs affect the hair cells of the cochlea. Unfortunately, these ototoxic effects may not become apparent during drug administration but may occur days to weeks after the therapy has been terminated. Ototoxicity may also be unilateral. Aspirin can produce a temporary hearing loss and tinnitus in individuals receiving high doses. In most cases, however, both of these symptoms disappear after aspirin use is terminated.
Loss Caused by Trauma
Etiology
Acquired sensorineural hearing loss caused by chronic, repeated exposure to loud sounds is common in the U.S. population. Four million people work each day in an environment with damaging levels of noise, and in 2008 approximately 2 million employees were exposed to workplace noise levels that put them at risk for hearing loss.9 Noise-induced hearing loss can be associated with the use of firearms, personal stereo systems, and power tools and with occupations such as firefighting, construction, agriculture, mining, manufacturing, transportation, and the military.
The loudness of sound/noise is measured in the logarithmic units of decibels (dB). A normal whisper is measured at approximately 30 dB, a conversation at 3 feet at 50 to 60 dB. In contrast, ambulance sirens have been measured at 120 dB, lawnmowers and motorcycles at 90 dB.10 Sounds exceeding 85 dB are considered potentially injurious, and chronic noise exposure is the most damaging. If exposure is severe enough, most structures of the inner ear can be damaged, including the organ of Corti. Sensory hair cells and supporting cells are lost because of overexposure. Noise-induced hearing loss typically is bilateral and affects higher (speech) frequencies first.3
Noise exposure has two phases: the first is a temporary threshold shift. When the ear is exposed to a loud sound, it will show a loss of sensitivity (a rise in the threshold for sound). If the hearing returns to normal after the sound has been removed, the shift was temporary and no permanent damage has occurred. If hearing does not return to normal, damage has occurred and the hearing impairment is permanent. Such a permanent threshold shift is the second phase of the damage. The ears of some individuals are more easily affected by noise, and considerable damage may occur before individuals are aware of the hearing loss.
Clinical manifestations
Individuals with hearing loss caused by noise trauma report that they are unable to discriminate words, particularly in noisy environments. Complaints about tinnitus are expressed more often than complaints about hearing loss. A diagnosis of noise-induced hearing loss is made through careful history and audiometric testing. Because noise-induced hearing loss is irreversible, no medical therapy can help once the problem has been established. Prevention is presently the only treatment for this type of hearing impairment.
Sensorineural hearing impairment can also occur with head trauma and subsequent damage to the structures of the inner ear. If blood is coming from the ear or the temporal bone is fractured, damage should be suspected. As a rule, hearing loss from trauma or head injury is permanent if the cochlea is damaged. A rare condition of sudden sensorineural hearing loss usually occurs in adults with middle ear problems. A viral or ischemic pathology is postulated. Prompt evaluation and treatment are necessary for these individuals. The prognosis is mixed but hearing may be recovered with intratympanic infusion of steroids.3
Presbycusis
Presbycusis is a sensorineural hearing loss and the most common form of hearing loss in older adults. Approximately 25% of people ages 65 to 75 and 50% of those older than 75 suffer from age-related hearing loss.3 Typically, the hearing impairment is of gradual onset, is bilateral, and results in difficulty hearing high-pitched tones and conversational speech. Presbycusis can progress to involve the middle and lower tones. Frequently, individuals complain that people are mumbling to them but deny any other type of hearing loss.
Etiology
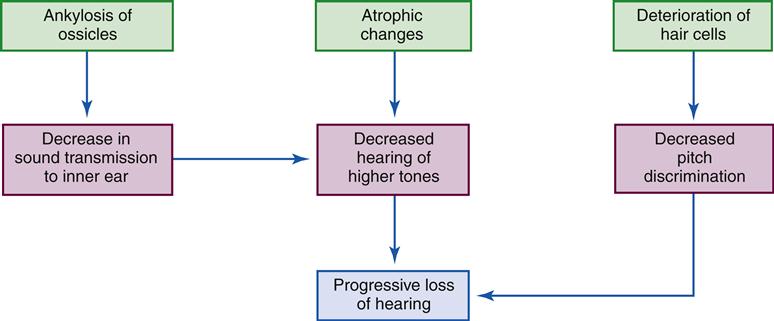
Four categories of presbycusis have been theorized: (1) sensory, characterized by atrophy and degeneration of the sensory and supporting cells; (2) neural, typified by loss of neurons in the cochlea and central nervous system; (3) metabolic, characterized by atrophy of the wall of the cochlea affecting central auditory processing; and (4) mechanical, in which the middle ear undergoes changes in properties with a resulting conductive hearing loss.3,8,11,12 Some of these age-related changes are shown in the Geriatric Considerations: Changes in Hearing box. An endless list of genetic, environmental, and disease states can also cause hearing loss in an older adult, many of which may occur concurrently. Thus uncertainty remains regarding the exact cause of presbycusis.
Diagnosis
Assessment of an individual with suspected presbycusis should begin with exclusion of all other causes of hearing impairment. Diseases such as diabetes, stroke, and heart disease may produce effects similar to those seen with hearing loss and must be ruled out. The diagnosis is made by obtaining a thorough history and performing audiometric studies. Individuals with presbycusis respond well to hearing aids that amplify sound. Many simple lifestyle adjustments that will be mentioned at the end of this section can dramatically improve the quality of life for an individual experiencing presbycusis. It is important in these patients to avoid excessive noise exposure and ototoxic drugs, which may cause further deterioration of hearing loss.8
Meniere Disease
Etiology and pathogenesis
Meniere disease is an excessive accumulation of endolymph in the membranous labyrinth. The volume of endolymph increases with distention of the scala media until the
membrane ruptures. Consequently, the neural end organs of the cochlea degenerate. Many conditions including allergies, viral and bacterial infections (such as syphilis), head trauma, metabolic derangements, and chronic stress have been suggested as causative agents, but the precise cause cannot be established in most cases.3,13 Men and women are equally affected by this disorder, and the onset of symptoms is typically in the fourth decade of life.13
Clinical manifestations
Clinical manifestations of Meniere disease include tinnitus, fluctuating sensorineural hearing loss, vertigo, and sensations of ear fullness. In the early stages, hearing loss fluctuates, with return to normal after the rupture heals. Remissions and exacerbations are typical. The hearing loss is usually in the low tones. As the disease progresses, hearing loss becomes permanent. Symptoms initially usually are unilateral; the mean time for the manifestation of bilateral symptoms is approximately 7 years.13
Episodes of vertigo may be immediately preceded by the sensation of pressure in the ear, increased hearing loss, increased tinnitus, or an alteration in the quality of these symptoms. The onset of vertigo is usually sudden, reaches maximal intensity within a few minutes, usually lasts for an hour or more, and either subsides completely or continues as a sensation of unsteadiness for several hours or days. The tinnitus is typically a low buzzing or blowing sound and is frequently louder before the attack of vertigo. The attacks are not precipitated by positional changes and may be several weeks or months apart. In the initial stages of the disease they may be years apart. If not treated, the episodes may become more frequent and severe. Nystagmus, which occurs only during acute attacks, may be directed to the side opposite the involved ear.
Diagnosis and treatment
Physical examination, including neurologic and otolaryngologic examination, is generally normal in those with Meniere disease. Radiologic studies are often used to rule out other causes of the symptoms of Meniere disease such as acoustic neuroma. Electrophysiologic studies, such as auditory brainstem response testing and electrocochleography, and audiometric tests as well as glycerol dehydration testing can lead to a diagnosis of Meniere disease.13 Caloric testing (irrigating the ears with warm and cool water) commonly reveals loss or impairment of thermally induced nystagmus on the involved side.13
Treatment for Meniere disease consists of providing symptomatic relief during acute episodes with antiemetics and anticholinergics such as meclizine. Between acute attacks, eating a low-sodium diet and using diuretics may help reduce the volume of endolymph. Cessation of smoking, management of stress, and elimination of caffeine from the diet are also suggested.
Several surgical interventions are used to manage Meniere disease. Shunts can be placed to drain excess endolymph, and ablation of portions of the eighth cranial nerve and destruction of the labyrinth are options. In refractory cases, patients may undergo intratympanic corticosteroid or gentamycin injections. These interventions have different indications, risks, and benefits associated with them. Almost all patients who choose surgical intervention have failed to respond to medical treatment.3
Otitis Media
Otitis media is an inflammation of the middle ear. It is almost always due to poor functioning of the eustachian tube and is often diagnosed by the presence of effusion. It is the most common reason for a child to require medical attention. In 2006 approximately 9 million children from newborns to 17 years of age were reported to have ear infections or otitis media.14
Otitis media is more common in the winter months when viral and bacterial infections are most prevalent. Upper respiratory tract infections can cause eustachian tubes to become blocked and predispose individuals to middle ear inflammation. Children are especially susceptible because of shorter, more flexible and horizontally positioned eustachian tubes. The dysfunction of the eustachian tube prevents middle ear secretions from draining and creates negative pressure in the middle ear space. Negative pressure leads to the introduction of infected nasopharyngeal secretions into the middle ear. Risk factors for otitis media include use of pacifiers, second-hand cigarette smoke exposure, gastroesophageal reflux, poor socioeconomic conditions, daycare attendance, and propped bottles. Males, Native Americans, Eskimo children, children with craniofacial abnormalities such as cleft palate, and individuals with Down syndrome have a higher incidence of otitis media.15
Much confusion surrounds the use of terminology in categorizing otitis media. This confusion relates to the presence of effusion and the length of illness (Table 46-1).
TABLE 46-1
COMPARISON OF OTITIS MEDIA TYPES
| TYPE | ONSET/DURATION | SYMPTOMS | TREATMENT OPTIONS |
| Acute otitis media | Sudden onset, associated with upper respiratory tract infections | Reddened tympanic membrane with poor mobility, may be bulging or ruptured, ear pain | Antibiotics, analgesics, antipyretics, or “watch and wait” |
| Recurrent acute otitis media | 3 or more episodes in 6 months | Same as above | Daily doses of prophylactic antibiotics in some cases, ventilation tube placement |
| Chronic otitis media | Duration of more than 12 wk, may develop as consequence of acute otitis media | Thick immobile tympanic membrane, purulent drainage from ear, may have conductive hearing loss, pain is rare | Removal of debris from middle ear, ventilation tube placement |
| Otitis media with effusion | May precede or follow any type of otitis media | Ear popping, feeling of pressure in middle ear, hearing loss, retraction of tympanic membrane, fluid line or bubbles | Treat acute otitis media or “watch and wait” |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree