5 Neoplasia
A neoplasm (‘new growth’) is a lesion that results from abnormal growth of a tissue, which is partly or completely autonomous of normal growth controls and persists after the initiating stimulus has been removed.
This chapter will describe how neoplasms form, how they are classified and how they behave.
CARCINOGENESIS
This section will describe the factors that predispose to the formation of neoplasms. Some examples of carcinogens are given in Table 5.1
Table 5.1 Examples of carcinogens
| Carcinogen | Neoplasm caused |
|---|---|
| Chemical: | |
| 3,4-benzpyrene (tobacco derivative) | Bronchogenic carcinoma |
| β-naphthylamine | Carcinoma of the bladder |
| Radiation: | |
| Ultraviolet light Ionising radiation | |
| Viruses: | |
| Human papilloma virus | Carcinoma of the cervix |
| Hepatitis B virus | Carcinoma of the liver |
| Others: | |
| Asbestos fibres | Mesothelioma |
| Aspergillus flavus aflatoxin | Carcinoma of the liver |
| Schistosoma | Carcinoma of the bladder |
CARCINOGENS
Chemical
A variety of chemicals have been identified as carcinogens in man, and others are suspected to be on the basis of their carcinogenic effects in experimental animals. There is no common structural link between the different types of chemical carcinogen, but they appear to have in common the ability to modify the structure of DNA: for example, by forming adducts or by adding alkyl groups.
The major classes of chemical carcinogens currently known are as follows.
Radiation
Ultraviolet light
The relationship between exposure to ultraviolet light and skin neoplasms is now well established. Neoplasms of the epidermis (basal cell carcinoma and squamous cell carcinoma), and the related precancerous condition solar/actinic keratosis, usually occur on sun-exposed sites and become more frequent with greater sun exposure. Similarly, malignant melanoma, a malignant neoplasm of melanocytes, is most common in fair-skinned individuals living in environments with high levels of sunlight exposure, such as white Australians. Malignant melanoma is uncommon in individuals of Afro-Caribbean origin because the greater density of melanin in their skin reduces the amount of ultraviolet light that reaches the melanocytes, which reside along the basal (deepest) layer of the epidermis. The pattern of ultraviolet exposure is important in determining which cells are most affected: long-term chronic expos-ure is associated with an increased risk of the development of basal cell carcinoma or squamous carcinoma, whereas melanoma is more strongly associated with episodes of ultraviolet exposure of sufficient intensity to cause sunburn.
Viruses
A growing number of viruses have been implicated in the development of neoplasms. There are manyexamples of virally induced neoplasms in animals, study of which has done much to promote our understanding of the molecular genetics of neoplasia. Virally induced neoplasms in humans are (as far as we are aware) rather less common. The most ubiquitous oncogenic viruses are the human papilloma viruses; other viruses with well-established carcinogenic effects are the Epstein-Barr virus and the hepatitis B virus (see Table 5.2).
| Virus | Neoplasm caused |
|---|---|
| Human papilloma virus | |
| Epstein-Barr virus | |
Mechanisms of viral carcinogenesis
Oncogenic RNA viruses are retroviruses which integrate their genetic material into the host genome using the enzyme reverse transcriptase. Although there are many examples of oncogenic retroviruses causingneoplasms in animals, this is rare in man. The best-known examples are Human T-Lymphotrophic Virus-1 (HTLV-1) which causes a form of lymphoma/leukaemia which is endemic in Japan and the Caribbean, and the human immunodeficiency virus (HIV). However, HIV probably does not have a direct carcinogenic effect; the neoplasms that are associated with HIV infection probably arise as a consequence of immunosuppression and may actually be caused by other types of virus. Thus, HIV infection may act as a cofactor for oncogenesis by other viruses. There are other examples of this phenomenon, such as the Epstein-Barr virus requiring malaria infection as a cofactor in the development of Burkitt’s lymphoma.
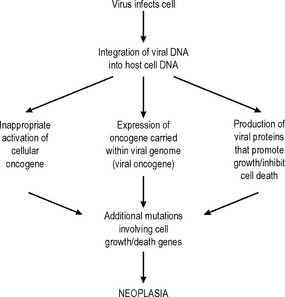
Other associations between viruses and neoplasms are being described – for example, herpes virus 8 and Kaposi’s sarcoma and myeloma – and it seems likely that further causative associations will be established in the future, particularly in neoplasms of the lymphoreticular system. The sequence of events by which viruses can cause neoplasia is outlined in Fig. 5.1.
Other biological factors
Helicobacter pylori infestation
There is a more direct link between Helicobacter infestation and a far less common neoplasm of the stomach, the so-called mucosa-associated lymphoid tissue (MALT) lymphoma. It has been shown that, despite having characteristics of a malignant neoplasm, such as clonality and invasiveness, MALT lymph-omas sometimes regress when patients are treated with Helicobacter-eradicating antibiotics. However, it is more likely that Helicobacter infestation represents a growth-sustaining stimulus, rather than a conventional carcinogen. These observations have led to some debate about whether MALT lymphomas are true neoplasms or not.
HOST FACTORS
Diet
The risks of developing neoplasia as a result of dietary contaminants are well illustrated by the example of aflatoxin-induced hepatocellular carcinoma. Other dietary factors may also modify the risk of developing certain neoplasms, for example, there is a link between high levels of dietary fat and breast carcinoma. The risk of colorectal carcinoma seems to be associated with diet, but is probably multi-factorial. Dietary fiber, fruit and vegetable consumption seem to be protective and red meat consumption seems to be deleterious, but it has been difficult to consistently show an independent effect for any of these factors. There is an increasing level of public interest in the importance of diet in causing or preventing cancers of many types. This has led to much interest in the media and even governmental public health campaigns, although the level of scientific evidence behind the benefits of any individual dietary manipulations is often dubious at best and imaginary at worst.
Genetic factors
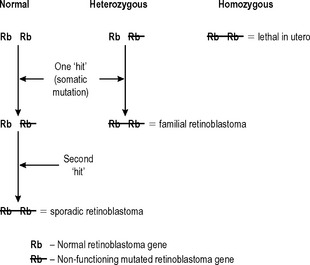
Changes in the structure and function of a cell’s genetic material are central to the development of neoplasia, and more than one such change is required in an individual cell before neoplasia can occur. If all of an individual’s cells already have an abnormality in a relevant gene as a result of that individual’s inherited genetic make-up (a germ-line mutation), then fewer subsequent changes are required for neoplasia to occur. This is well illustrated by the rare familial retinoblastoma syndrome (Fig. 5.2). If both alleles of the retinoblastoma (Rb1) gene in an individual retinal cell are non-functional, retinoblastoma can develop from that cell. Sporadic retinoblastoma is a rare tumour because it is unusual for both retinoblastoma alleles in an individual cell to acquire mutations that inhibit their function. However, if one allele is already non-functional because it was inherited in a defective form, then the chances of retinoblastoma developing as a result of a subsequent mutation of the other allele are very high. This also demonstrates that the ‘retinoblastoma’ gene is a tumour suppressor gene. This is a common property of the genes that are abnormal in the various familial cancer syndromes.
Another characteristic that the retinoblastoma syndrome shows, that is common in familial cancer syndromes, is that it affects more than one tissue. If individuals with the retinoblastoma syndrome survive the development of retinoblastomas (which are usually bilateral) early in childhood, they have a very high incidence of osteosarcoma during adolescence. The retinoblastoma syndrome is used here as an illustration because its genetics are simple and well characterised. However, there are a number of other familial cancer syndromes, many of which, such as familial polyposis coli, are more common. Increasingly, these syndromes are being identified with mutations in genes that are involved in DNA repair such as the BRCA1 gene associated with familial breast cancer. The best known examples are given in Table 5.3.
Table 5.3 Examples of familial cancer syndromes
| Syndrome | Gene affected | Resultant neoplasms |
|---|---|---|
| Li Fraumeni | p53 | Breast, ovarian carcinomas, astrocytomas, sarcomas |
| Retinoblastoma | Rb1 | Retinoblastoma, osteosarcoma |
| Familial polyposis coli von Hippel-Lindau | APC | GI tract carcinomas, mainly colon |
| VHL | Renal carcinoma, phaeochromocytoma, haemangioblastoma | |
| Multiple endocrine neoplasia syndromes (I–III) | RET, others | Tumours of pituitary parathyroids, thyroid, pancreas, adrenal (combination depends on which syndrome) |
| Familial breast cancer | BRCA 1, BRCA 2 | Breast, ovarian syndrome prostatic carcinomas |
Immune response
The host immune response has another important indirect effect on the development of neoplasms. There is a strong positive link between immunodeficiency and the development of neoplasia. This is illustrated by the frequency of development of lymphomas and Kaposi’s sarcoma in the acquired immune deficiency syndrome (AIDS), and cutaneous and anogenital squamous carcinomas in organ transplant recipients taking immunosuppressive therapy. In these settings the increased risk of neoplasia seems to be due to an inadequate immune response to oncogenic viruses such as HHV-8 in the case of Kaposi’s sarcoma and HPV in the case of transplant-associated squamous carcinomas.
PREMALIGNANT DISEASE
(The distinction between benign and malignant neoplasms will be defined below. The term dysplasia was defined in Chapter 4.)
Malignant change in benign neoplasms
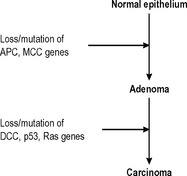
The majority of benign neoplasms do not alter in any way, but some benign neoplasms have the ability to progress to become malignant neoplasms. Probably the best-characterised example of this phenomenon is the adenoma-carcinoma sequence in the colon. Adenomatous polyps of the colon are more numerous than colonic carcinomas, but all adenomatous polyps have the potential to develop into carcinomas, and many (but not all) carcinomas originate from adenomatous polyps. The polyps most likely to undergo malignant change show the greatest degree of histological dysplasia and a sequence of genetic changes that leads to the development of colorectal carcinoma from normal epithelium via adenomatous polyps has now been described (Fig. 5.3).
Metaplasia-dysplasia sequence
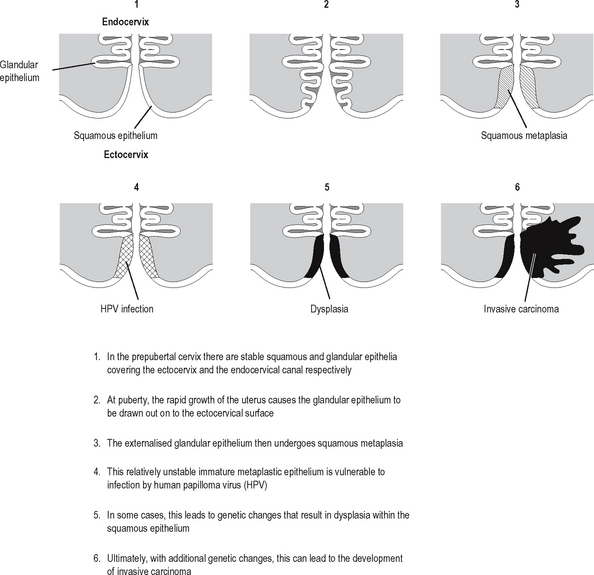
Neoplastic transformation of cells occurs in cells undergoing proliferation, and is particularly likely to occur if the cells are also undergoing metaplasia (defined and described in the previous chapter). Neoplastic transformation of metaplastic epithelium usually follows a predictable and histologically identifiable sequence of low grade dysplasia progressing to high grade dysplasia/in-situ malignancy to invasive malignancy as additional genetic abnormalities are acquired in the neoplastic population. This progression is very well demonstrated in the cervix (Fig. 5.4). Other examples of the metaplasia–dysplasia sequence are shown in Table 5.4.
Table 5.4 Examples of the metaplasia-dysplasia sequence
| Organ | Form of metaplasia undergoing dysplasia | Resulting malignancy |
|---|---|---|
| Oesophagus | Barrett’s oesophagus (intestinal metaplasia) | Oesphageal adenocarcinoma |
| Stomach | Intestinal metaplasia (associated with achlorhydria) | Gastric adenocarcinoma |
| Bronchus | Squamous metaplasia | Brochogenic squamous carcinoma |
| Cervix | Squamous metaplasia | Cervical squamous carcinoma |
Premalignant conditions
These are usually conditions characterised by high cell turnover over a sustained period of time, usually resulting from a destructive form of chronic inflammation. Congenital abnormalities can also be premalignant conditions: for example, maldescent of the testis is associated with an increased risk of testicular neoplasia in later life. Some examples of premalignant conditions are given in Table 5.5.
Table 5.5 Examples of premalignant conditions
| Premalignant condition | Resulting neoplasm |
|---|---|
| Ulcerative colitis | Colorectal carcinoma |
| Chronic fistulae | Squamous carcinoma |
| Epithelial hyperplasia of the breast | Breast carcinoma |
| Paget’s disease of bone | Osteosarcoma |
| Xeroderma pigmentosum | Skin malignancies |
CARCINOGENIC PROCESS
The precise chains of molecular events in most tumour types have yet to be established.