Mumps Virus
Steven A. Rubin
Christian J. Sauder
Kathryn M. Carbone
History
In the 5th century BC, Hippocrates described a mild epidemic illness associated with nonsuppurative swelling near the ears and, variably, with painful swelling of one or both testes. These descriptions of parotitis and orchitis, respectively, are the hallmarks of mumps virus (MuV) infection. The name mumps may derive from an old English verb that means to grimace, grin, or mumble. Hamilton, a physician of the late 18th century, is credited as being the first to associate central nervous system (CNS) involvement with mumps in his description of the neuropathology of a fatal case. Later studies would reveal MuV as a highly neurotropic agent and a leading cause of virus-induced aseptic meningitis and encephalitis.20,42,190
A number of laboratory investigations suggested that a filterable, transmissible agent was responsible for mumps141,281,423; however, a viral etiology was not proven until 1935 when Johnson and Goodpasture, using bacteria-free parotid secretions, successfully transmitted the disease between monkeys and children and then back to naïve monkeys, fulfilling Koch’s postulates.186,187
The demonstration by Habel145 and Enders108 in 1945 that MuV could be isolated and propagated in embryonated eggs enabled the demonstration of the hemagglutinating, hemolytic,268 and neuraminidase182 properties of the virus, leading to the development of an inactivated vaccine in 1946144 and to the first live virus vaccine in 1958.353 The introduction of tissue culture as a practical alternative for the propagation and study of the virus in 1948414 was pivotal for advancing studies of the epidemiology and pathogenesis of the disease as well as the molecular biology of the virus, permitting the development of cell-based vaccines.
Although historically a benign disease of childhood, mumps was viewed as a major concern for the military, particularly in times of mobilization. Mumps was a notable issue during the Civil War of the United States,166 World War I,138 and during World War II,253,302 and continues to occur in military settings.13,218,316 During World War I, mumps was a leading cause of days lost from active duty in the United States Army in France, exceeded only by losses due to influenza and gonorrhea infections.
Infectious Agent
Classification
MuV is a nonsegmented, negative-strand RNA virus in the family Paramyxoviridae, subfamily Paramyxovirinae, genus Rubulavirus. See Chapter 33 for a detailed overview of the Paramyxoviridae.
Virion Morphology and Structure
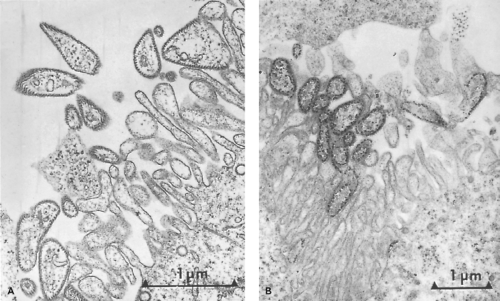
Mumps virions are pleomorphic particles ranging from 100 to 600 nm in size, consisting of a helical ribonucleoprotein (RNP) core surrounded by a host cell–derived lipid envelope (Fig. 35.1). The RNP consists of a single-stranded RNA
(ssRNA) molecule coated by the viral nucleoprotein.82 The RNP appears to be a hollow tube with a unit length of approximately 1 mm, a diameter of 17 to 20 nm, and a central core of 5 to 6 nm.168,169,251 The viral host cell–derived envelope contains the viral glycoproteins that project 12 to 15 nm from the virion surface.
(ssRNA) molecule coated by the viral nucleoprotein.82 The RNP appears to be a hollow tube with a unit length of approximately 1 mm, a diameter of 17 to 20 nm, and a central core of 5 to 6 nm.168,169,251 The viral host cell–derived envelope contains the viral glycoproteins that project 12 to 15 nm from the virion surface.
Full-length genomic RNA (gRNA) is an unsegmented, single-stranded macromolecule of negative polarity that consists of 15,384 nucleotides. The presence of multiploid virions in MuV preparations has been reported, but only one of the genomes is believed to be biologically active.250
Genomic Organization
The MuV genome contains 7 tandemly linked transcription units: the nucleo- (N), V/phospho-/I (V/P/I), matrix (M), fusion (F), small hydrophobic (SH), hemagglutinin-neuraminidase (HN), and large (L) protein genes. The gene order is 3′-N-V/P/I-M-F-SH-HN-L-5′.105,107 The MuV genome is flanked at the 3′ end by an extracistronic leader sequence of 55 nucleotides370 and at the 5′ end by a trailer sequence of 24 nucleotides,288 of which the last 10 share inverse complementarity. These regions are essential for transcription and replication. Unlike most other paramyxoviruses, MuV does not have identical gene-start and gene-end sequences. The consensus gene-start sequence is 3′-U-U/C-C-G/U-G/U-N-C/U-U/C-U and that of the stop sequence is 3′-A-A/U-U/A-U-C/A-U6-7,105 separated from each other by intergenic sequences of 1 to 7 nucleotides.
Each gene encodes a single protein, with the exception of the V/P/I gene (conventionally referred to as the P gene), which gives rise to additional mRNA species as a result of the cotranscriptional insertion of nontemplated G nucleotides between positions 461 to 466.107,297 Faithful transcription of the gene produces the V protein (formerly referred to as NS1 protein), whereas insertion of two G residues within the editing site produces an mRNA encoding the P protein and insertion of one or four G residues produces an mRNA encoding the I protein (formerly referred to as NS2 protein and analogous to the W protein reported in related paramyxoviruses). Thus, the V, P, and I proteins have the same amino-terminal segment but different C-terminal regions.
Genome transcription occurs by a stop-start mechanism in which the viral polymerase produces a decreasing gradient of monocistronic mRNA for genes located further from the 3′-end promoter (see Chapter 33). Due to the occasional failure of the viral RNA–dependent RNA polymerase (RdRp) to recognize the intragenic stop signals, bi-, tri-, tetra-, penta- and hexacistronic read-through transcripts can be detected in MuV-infected cells, from which only the first cistron is translated.5,89,106 The size of the monocistronic mRNAs as well as the number of amino acids and molecular weights of the MuV proteins are provided in Table 35.1.
Virus Proteins and Replication
N, P and L Proteins
The first translated transcriptional unit of the virus, the N protein, complexes with the gRNA to form the RNP, the template for RNA synthesis. Only encapsidated RNA, not naked
RNA, can be transcribed. RNA synthesis begins with the binding of the RdRp, a complex of the P and L proteins, to the RNP, as inferred from other studies of highly homologous viruses.140,147
RNA, can be transcribed. RNA synthesis begins with the binding of the RdRp, a complex of the P and L proteins, to the RNP, as inferred from other studies of highly homologous viruses.140,147
Table 35.1 Mumps Genes and Gene Products | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The MuV N protein is a bipartite molecule consisting of a globular N-terminal assembly domain (amino acids 1 to 398) mediating RNA binding (to form the RNP), and an unstructured hypervariable C-terminal tail believed, based on studies of related viruses, to interact with the MuV M protein during virion assembly.35,88,342
The MuV P protein forms homotrimers via a centrally located coiled-coil domain87 and complexes with the L protein to form the RdRp. The C-terminal 48 amino acids of the P protein is a nucleocapsid-binding domain (NBD), responsible for tethering of the RdRp to the RNP by binding to the assembly domain of the N protein (amino acids 1 to 398).207 The three-dimensional structure of the NBD has been deciphered using X-ray crystallography and revealed formation of a compact bundle of three α-helices, a feature that appears to be conserved among paramyxovirinae.208 Unlike the NBD of measles virus and Sendai virus, that of MuV lacks defined tertiary structure and is fundamentally unstable.207 As indicated by its name, the MuV P protein is heavily phosphorylated,278 an action believed to regulate polymerase activity, but this has not been clearly demonstrated for MuV.123,361,362
Six functional domains have been identified in the MuV L protein based on sequence similarity with L proteins from related viruses.308,350 These domains have been ascribed all catalytic functions such as execution of transcription and replication, as well as methylation, capping, and polyadenylation of mRNAs (see Chapter 33). A detailed functional and structural analysis of the MuV L protein has not yet been carried out.
Matrix Protein
The MuV M protein orchestrates virus assembly and budding. Only the MuV M protein when expressed alone is sufficient for virus-like particle production, although in the absence of co-expression with other viral proteins, efficiency is low.234 Evidence indicates that the M protein binds to the cytoplasmic tails of the F and HN glycoproteins assembled at distinct locations on cellular membranes, presumably lipid raft microdomains. There, the M protein functions as an adapter, physically linking the region of host-cell membrane expressing the viral F and HN glycoproteins with the viral RNP via its interaction with the N protein.152,234 Budding appears to be mediated by an interaction between the MuV M protein and the cellular endosomal sorting complex for transport (ESCRT) machinery.234 The MuV M protein has also been shown to interact with host proteins angiomotin-like 1 (AmotL1) and 14-3-3.298,299 Whereas the AmotL1–M protein interaction appears to promote MuV VLP production, based on studies of PIV5, M protein interaction with 14-3-3 decreases the efficiency of virus budding. Interestingly, the MuV M 14-3-3 binding site is adjacent to a sequence motif conserved among rubulaviruses, presumably functioning as a binding site for the host protein caveolin 1 (Cav-1), an essential structural component of caveolae that are considered a subset of lipid rafts. It is therefore likely that MuV budding, like that of PIV5, occurs from caveolae. The close proximity of the 14-3-3 and postulated Cav-1 binding sites raises the possibility that the MuV
M protein switches between either binding to 14-3-3 or Cav-1, thereby regulating the amount of M that can participate in virus budding.299
M protein switches between either binding to 14-3-3 or Cav-1, thereby regulating the amount of M that can participate in virus budding.299
Surface Glycoproteins
The F and HN are transmembrane glycoproteins of types I and II, respectively. The F glycoprotein is synthesized as an inactive precursor, F0, that is targeted to the rough endoplasmic reticulum via a 19 amino acid signal peptide, which is subsequently cleaved.408 Following N-glycosylation, the precursor is transported to the trans-Golgi network where it is proteolytically cleaved between amino acids 102 and 103 by the host cell protease furin at the R-R-H-K-R motif to produce two disulfide-linked heterodimers F1 and F2.162,257,278,409 Cleavage of F0 is essential for virus-to-cell and cell-to-cell membrane fusion and for virus infectivity. The amino terminus of the F1 subunit possesses the fusion peptide, a conserved hydrophobic domain exposed by the cleavage event (see Chapter 33). Evidence indicates that a second cleavage event occurs during which F1 is processed into two subunits, F1a and F1b; this event is important in mediating fusion activity.400
At least two heptad repeat (HR) domains are found in the F1 ectodomain: HR1 at the amino terminus adjacent to the fusion peptide and HR2 at the carboxyl terminus adjacent to the transmembrane domain.239 The MuV F protein forms homotrimers and the HR1 and HR2 domains interact to form a stable six-helix bundle structure.239,249 The HR2 domain is also involved in the binding of F with HN. In related viruses, additional HR domains have been identified, although this has not been confirmed for MuV.131,270 The specific processes involved in MuV fusion have not been delineated; however, based on similarity with the six-helix bundle structural of the PIV5 F protein, the events that mediate MuV fusion are likely similar to those for PIV5 (see Chapter 34).
In its native state, the HN protein is a disulfide-bonded oligomer assembled as homotetramers. The protein is held in the lipid bilayer by a hydrophobic domain of 19 residues near the amino terminal, a domain that probably also serves as a signal sequence in a manner similar to other paramyxovirus HN proteins. HN monomers display a membrane proximal stalk that supports a globular head, forming a six-blade propeller structure.84,219 The globular head is responsible for attachment and neuraminidase activity182,291; the stalk region, in conjunction with the F protein, mediates virus-to-cell and cell-to-cell fusion.371,382 Based on inference from related viruses, a single site is believed to mediate both neuraminidase and receptor binding activity using a protein conformational switch mechanism to toggle activity.77,84,226 There may also be a second sialic acid binding site within the globular head region, theorized to stabilize viral–cellular membrane interactions in addition to the large binding pocket that fluxes between neuraminidase and receptor binding activity.37,314,437 Two competing models on the HN-assisted mechanism of F protein activation have emerged78 (see Chapter 34 for details). Briefly, the “clamp model” proposes that the HN protein associates with F intracellularly, stabilizing the prefusion conformation of the protein. Upon binding of HN protein to its receptor, the F protein is released, allowing for the conformational change required for fusion activity. In the “provocateur model,” a change in the structure of the HN itself is postulated to promote fusion activity. In this model, the HN protein either is preassociated with the F protein or associates with the protein following receptor binding, at which point the HN protein undergoes a conformational change leading to destabilization of the F protein, which confers fusion activity. Results from recent studies using PIV5 HN and F proteins support the “provocateur model.” Whether this model applies to MuV awaits confirmation.
V Protein
The MuV V protein, as reported for other rubulaviruses, is involved in inhibiting IFN production and signaling (see Chapter 33). The 69 aa C-terminal cysteine-rich domain of the MuV V protein appears to be the key player in these activities. This region directly interacts with MDA5 (melanoma differentiation–associated gene 5), a pattern recognition receptor that recognizes cytosolic viral RNA, and with the TBK-1 (TANK-binding kinase 1)/IKKε (inhibitor of kB kinase-ε) kinases responsible for interferon regulatory factor-3 (IRF-3) phosphorylation. MuV V protein interaction with MDA5 inhibits its ability to induce transactivation of the IFN-β promoter,12,320 and in the case of TBK-1/IKKε, leads to their ubiquitination and subsequent proteasomal degradation,243 preventing IRF-3 phosphorylation, an event required for transcription of IFN and IFN-stimulated genes. The C-terminus of the MuV V protein also interacts with the cellular signal transducer and activator of transcription (STAT) proteins, STAT-1, STAT-2, and STAT-3.151,282,434,435 STAT-1 and STAT-2 play a central role in the IFN signal transduction pathway that eventually leads to activation of IFN-induced genes. STAT 3 has also been implicated in cellular antiviral responses.318,389 Binding of MuV V leads to the ubiquitination and subsequent degradation through the proteasomal pathway of STAT-1 and STAT-3, but not of STAT-2; however, the latter is required for targeting of STAT-1 for degradation.216,388,389,435 The C-terminus of the MuV V protein is not the only region important in these interactions, as exemplified in a study demonstrating that a single point mutation at amino acid position 95 abrogates the ability of the MuV V protein to degrade STAT3, while retaining the virus’s ability to target STAT 1.318
The observation that the MuV V protein can oligomerize and form spherical particles suggests that the MuV protein provides a scaffold for coordinating the assembly of the cellular components (e.g., UV-damaged DNA binding protein 1 [DDB1], cullin 4A [Cul4A], and regulator of cullin 1 [Roc1]) involved in ubiquitination, collectively referred to as V-dependent degradation complexes (VDC).388 For more details on VDC, see Chapter 33.
SH Protein
The SH protein consists of 57 amino acids, 25 of which are highly hydrophobic and clustered at the amino terminus serving as a membrane anchor region with its C-terminus facing the cytoplasm.104,106,367 The predicted SH mRNA exhibits two AUG start codons, the second of which represents the actual AUG for the SH open reading frame. The first AUG, located at positions 4 to 6, gives rise to a minicistron with a stop codon at nucleotides 19 to 21.106,367 Due to the immediate proximity of the first cistron to the cap of the mRNA, it is predicted that with a frequency of about 50%, the ribosomes will skip the first AUG and initiate at the second cistron,212 thus enabling translation of the SH protein, although at reduced efficiency. The
biological significance of this minicistron and its proposed role in reducing the amount of SH protein synthesized is unknown.
biological significance of this minicistron and its proposed role in reducing the amount of SH protein synthesized is unknown.
In certain MuV strains, such as the Enders and Rubini strains, a point mutation exists in the putative F gene polyadenylation signal resulting in an F-SH bicistronic mRNA,220,367,368 from which only the F protein is made. This demonstrates that the SH protein is not essential for virus replication, which has been confirmed for PIV5.156
Studies of PIV5 demonstrated that the SH protein facilitates evasion of the host antiviral response via blocking the TNF-α-mediated apoptosis pathway.156,157 The MuV SH protein appears to be functionally similar, based on recombinant DNA studies in which PIV5 SH gene was then replaced with that of MuV.418 Yeast two-hybrid and co-immunoprecipitation studies identified ataxin-1 ubiquitin-like interacting protein (A1Up) as a cellular target of the MuV SH protein. This protein plays a role in proteasomal degradation, but the biological significance of its interaction with the MuV SH protein is not clear.427
I Protein
Expression of the I protein in infected cells was confirmed, but its role in the life cycle of the virus is unknown.297
Virus Infection of Host Cells
Sialic acid, an acyl derivative of neuraminic acid, is found on cellular glycoproteins and lipids and serves as the receptor for MuV. Following attachment of the virus to its receptor, the viral and cellular membranes fuse, permitting entry of the viral RNP followed by its transcription and replication. These events occur in a manner common to most paramyxoviruses (see Chapter 33 for a detailed description of these and other events such as viral assembly and release).
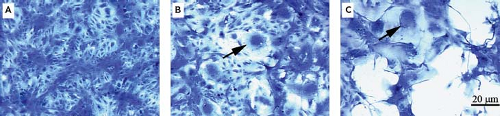
Because the MuV receptor is ubiquitously expressed on mammalian cells, the virus infects most cell types. The presence of MuV is typically detected in cell cultures by the induction of syncytia, large homogeneous masses of cytoplasm enclosing numerous nuclei (Fig. 35.2), followed by lysis of infected monolayers; however, the cytopathic effects of MuV can vary considerably among isolates and substrates, and in some instances, there is little evident morphologic change.4,142,250 The type of cytopathic effect produced in vitro does not correlate with the in vivo behavior of the virus.
Pathology and Pathogenesis
Infection in Experimental Animals
Humans are the only natural host of MuV, although experimental infection has been induced in laboratory animals, including monkeys,230,327,334 hamsters,192,295,419 mice,205,295 and rats.315,331 Experimental infection of animals has mostly been used to study the pathogenesis of MuV neurotropism and neurovirulence. Intracerebral inoculation of MuV into the suckling hamster results in a massive inflammatory response, including meningitis, encephalitis, and ventriculitis. Intraperitoneal inoculation of virulent strains into suckling hamsters also leads to CNS infection.420 Associated neuropathology includes hydrocephalus, Chiari type I cerebellar malformation, and neuronal necrosis.204,295,366,369,421 Many of these are reported features of CNS infection in humans, suggesting the applicability of the hamster model for studying the pathogenesis of MuV infection in man. Similar findings have also been reported in monkeys.70,241,326,334,436 Very little, if any, work has been accomplished in mice because MuV infection in this species tends to be abortive, thereby limiting the value of this model system.155,317,383 Early studies also indicated mumps infection to be abortive in rats, unless adapted by serial passage in brain.315 Subsequent studies, however, determined that intracerebral inoculation of the virus into newborn rats resulted in inflammation of the ventricular system (choroiditis, ependymitis) and hydrocephalus, but not meningitis or encephalitis.331 Interestingly, the severity of hydrocephalus in rats was found to correlate well with the virus strain-specific neurovirulence potential for humans, suggesting the relevance of such a model of disease in examining the pathogenesis and molecular basis of MuV neurovirulence.332 The severity of virus-induced neuropathology in marmosets, but not other monkey species, was also found to correlate with virus neurovirulence potential for humans.328
Infection in Humans
Transmissibility after nasal or buccal mucosal inoculation of virus187 suggests that natural infection is initiated by droplet spread. The incubation period is 16 to 18 days,167,259 during which the virus multiplies in the upper respiratory mucosa before spreading to draining lymph nodes. Based on studies involving experimental infection of hamsters, virus disseminates via a transient plasma viremia,420 potentially infecting multiple tissues and
organ systems.172 The most common sites of virus dissemination are glandular tissues (parotid glands, testes, breasts, and pancreas), and the CNS. If viruria is used as an indication of kidney infection, then kidney involvement is common in mumps, although clinical nephritis is rarely diagnosed.392,394 In rare cases, MuV can be transmitted transplacentally.
organ systems.172 The most common sites of virus dissemination are glandular tissues (parotid glands, testes, breasts, and pancreas), and the CNS. If viruria is used as an indication of kidney infection, then kidney involvement is common in mumps, although clinical nephritis is rarely diagnosed.392,394 In rare cases, MuV can be transmitted transplacentally.
Virus is shed in saliva as early as 6 days before the onset of parotitis.159 Termination of viral shedding correlates with the local appearance of virus-specific secretory IgA and IgM, as early as a few days after disease onset.67,304 Fewer than 15% of patients continue to shed virus beyond day 4 of symptom onset.310 Thus, patients with mumps are capable of spreading virus by the respiratory route over a 10-day interval.
Plasma viremia disappears coincident with the development of MuV–specific antibody, which can be detected in serum as early as 11 days after infection of humans.159 Animal models suggest that circulating infected lymphocytes provide a means for the spread of virus in the face of mounting humoral immunity.420 Despite the apparent high frequency of viremia during mumps, MuV has only rarely been detected in blood.183,201,294
Parotid Gland
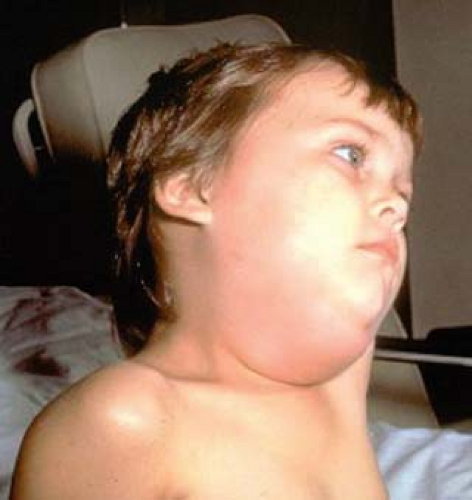
Initial clinical symptoms usually relate to infection of the parotid gland, but viral involvement of this gland is neither a primary nor obligate step in the infection.202 Virus infects the ductal epithelium, resulting in desquamation of involved cells, periductal interstitial edema, and a local inflammatory reaction primarily involving lymphocytes. Swelling, inflammation, and tissue damage in the parotid gland can produce elevation of serum and urine amylase levels.346
Central Nervous System
MuV CNS invasion, as demonstrated by cerebrospinal fluid (CSF) pleocytosis, occurs in greater than one third of patients presenting with clinical mumps.20,42,46,112 Symptomatic CNS infection (i.e., meningitis) is less common, occurring in approximately 10% of cases.20,149,198 Encephalitis occurs in less than 0.5% of cases. In the prevaccine era, MuV was a leading cause of viral meningitis and encephalitis in most developed countries and continues to be a leading cause in unvaccinated populations worldwide.18,125,160,258 Neurologic manifestations appear with a 3:1 or greater male–female ratio30,210,228 and are generally preceded by parotitis by 4 to 5 days but can occur before or in the total absence of detectable salivary gland swelling.
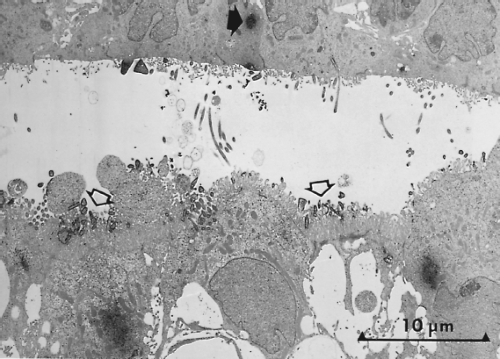
As inferred from animal studies, viral invasion of the CNS occurs across the choroid plexus.420 Blood-borne infected mononuclear cells, and possibly cell-free virus, can cross the fenestrated endothelium of the choroid plexus stroma and serve as a source for subsequent infection of the choroidal epithelium. Maturation of virus from the ventricular surfaces of choroidal cells provides progeny virions that are widely distributed through ventricular pathways and the subarachnoid space by CSF (Fig. 35.3). Virus can penetrate brain parenchyma and infect neurons by contiguous spread from infected ependymal cells that line the ventricular cavities of the brain. Once within neurons, virus most likely spreads along neuronal pathways, as reported in nonhuman primates.230 Although primary encephalitis is typically the response to direct viral invasion of neural cells, cases of postinfectious encephalitis, an autoimmune attack on CNS myelin sheaths, also occurs. Symptoms of primary encephalitis appear before or during the development of parotitis; symptoms of postinfectious encephalitis and associated demyelination appear 1 to 3 weeks after the onset of parotitis.238,372
Typical CNS pathology of MuV encephalitis includes edema and congestion throughout the brain with hemorrhage, lymphocytic perivascular infiltration, perivascular gliosis, and demyelination. Findings in the spinal cord include early degenerative changes in the anterior horn cells and perineuronal edema.102,264,372 When seen, the selective periventricular myelin loss with relative sparing of axons is typical of parainfectious autoimmune encephalitis.
Rarely is CNS infection fatal and most cases resolve without sequelae. In some instances, however, electroencephalographic changes, ataxia, and behavioral disturbances may take months to resolve173,210 and permanent neurologic damage—such as obstructive hydrocephalus,286 deafness,236 and myelitis284,395—can occur.
Hydrocephalus can develop days to years after initial MuV infection and can lead to progressively worsening headaches, mental status changes, and gait abnormalities.71,137,286 The pathogenesis of hydrocephalus is inferred from animal studies that have suggested that desquamation of virus-infected ventricular ependymal cells blocks egress of CSF through the aqueduct of Sylvius. Abnormally restricted flow of CSF to adsorptive sites over the cerebral convexities results in progressive enlargement of the lateral and third ventricles.191,192 The presence of ependymal cell debris in the CSF of humans presenting with MuV CNS infection suggests a similar mechanism of hydrocephalus induction in humans.161,286,380 Hydrocephalus, however, has also been observed before, or in the total absence of, aqueductal stenosis.189,364,365,419 This indicates that stenosis of the aqueduct could be a secondary consequence of external compression by surrounding edematous tissue and not causally related to the pathogenesis of hydrocephalus.
MuV is the most frequent cause of acquired sensorineural hearing loss in children. Transient, high-frequency deafness is the most common form, occurring in approximately 4% of mumps cases.402 Permanent deafness occurs in less than 1 per 20,000 cases27,153 and is usually unilateral. Deafness is believed to be the result of direct viral invasion of the cochlea, likely via the perilymph, which freely communicates with the CSF146,254,352,415; however, evidence indicates a hematogenous route exists.265 Pathology includes degeneration of the stria vascularis, tectorial membrane, and organ of Corti and collapse of Reissner’s membrane.111 Hearing loss caused by indirect effects of virus infection (e.g., immune-mediated damage) have also been suggested.390 Deafness is not disproportionately seen with other complications, suggesting no specific pathogenic link with parotitis, meningitis, or other complications. Deafness has also been observed in otherwise asymptomatic patients.287,390
Gonads
Orchitis, usually unilateral, occurs in approximately 20% of postpubertal men who develop mumps.16,124,224 Orchitis rarely occurs in children, suggesting that certain hormonal factors, such as receptors for luteinizing hormone and follicle-stimulating hormone expressed during adolescence, might promote testicular tropism of the virus.376 Virus has been isolated from testicular biopsies of the affected gland within the first 4 days of symptoms, and from semen,29,179 strongly suggesting that symptomatic gonadal involvement reflects local virus replication. The
seminiferous tubules may be the primary site of viral replication, with local lymphocytic infiltration and edema of interstitial tissues.
seminiferous tubules may be the primary site of viral replication, with local lymphocytic infiltration and edema of interstitial tissues.
Kidneys
Based on the frequency of viruria, virus frequently disseminates to the kidneys, where epithelial cells of the distal tubules, calyces, and ureters appear to be primary sites of virus replication.413 Viruria can be detected in most patients, sometimes for as long as 14 days after the onset of clinical symptoms.392,393 Mild abnormalities of renal function have been described, but they are usually of little clinical importance.392 Although virus dissemination to, and replication in, the parotid gland and kidney can occur simultaneously, replication in renal tissue is more prolonged and continues well beyond the appearance of neutralizing antibody in serum.
Pancreas
Pancreatic involvement, diagnosed in 1% to 27% of cases,38,47,64,232,296,345,356,376,416 is usually expressed as mild epigastric pain, but severe hemorrhagic pancreatitis114 and transient exocrine function abnormalities92 have been reported. MuV infects human pancreatic beta cells in vitro,403 and virus infection of the pancreas has been demonstrated in hamsters inoculated intraperitoneally.420 Viral infections have been considered a possible precipitating event leading to the onset of about one third of all cases of juvenile-onset or type I insulin-dependent diabetes mellitus (IDDM); however, whether MuV causes IDDM is unclear.83,127,136,344 No association has been found between mumps and type II diabetes.240
Heart and Joint Tissues
Myocardial invasion occurs frequently in mumps, as indicated by electrocardiographic abnormalities.14 Although it is seldom symptomatic, interstitial lymphocytic myocarditis and mild pericarditis may occur following mumps replication in cells of the myocardium and pericardium.43 MuV myocarditis can lead to the rare but serious sequelae of endocardial fibroelastosis.279
Mild to moderately severe mono- or polyarticular and, often, migratory arthritis has rarely been associated with mumps.139 MuV has not been isolated from joint fluids or synovial tissues, and no evidence exists for significant immune complex deposition.
Fetus and Newborn
MuV can be transmitted transplacentally as demonstrated in nonhuman primates357 and by the isolation of the virus from the human fetus following spontaneous first-trimester abortion during maternal mumps.221,433 The virus can produce a fetal wastage in humans, with or without subsequent spread of virus to involve fetal tissues directly.433 MuV has also been isolated from fetal tissues following planned therapeutic abortion of seronegative women 1 week after vaccination with live, attenuated MuV, although it is unclear if the virus detected was vaccine virus, or wild-type virus coincidentally contracted at or shortly before the time of vaccination.430 A proliferative necrotizing villitis with decidual cells containing intracytoplasmic inclusions has been described in the products of spontaneous and induced abortions.129 Late-gestation intrauterine infection was reported in an infant born to a mother who developed mumps more than 4 weeks before delivery, diagnosed by reverse transcription polymerase chain reaction (RT-PCR) testing of the infant’s cord blood cells.363 This infant developed severe pulmonary symptoms, including hypertension and hemorrhage.
MuV is excreted in breast milk,203 but few cases of perinatal mumps have been described195,222 and it is not clear if breast milk was responsible for these cases. There appears to be a somewhat different mode of pathogenesis of mumps in newborns. In the first year or two of life, infants may have only pulmonary involvement without evidence of parotitis.195,363 Split immunologic recognition in the infants can follow maternal parotitis, resulting in MuV-specific cell-mediated immune responses without a concomitant antibody response.1,357,378
Molecular Basis of Virulence
While comprehensive studies on the molecular basis of MuV virulence have yet to be performed, it is clear that the genetic basis of MuV neurotropism and neurovirulence does not lie within any one gene7,329,339,348,428 and no simple pattern of genomic mutations capable of discriminating virulent from attenuated MuV strains has been identified.8,177,339
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree