Human T-Cell Leukemia Virus Types 1 and 2
Masahiro Fujii
Masao Matsuoka
History
The discovery of oncogenic retroviruses in animals led to a search for retroviruses in human cancer cells. In 1980, the first human retrovirus was found in a T-cell line established from a patient with mycosis fungoides, and the retrovirus was named human T-cell leukemia virus (HTLV).85,296 Prior to this discovery, the disease entity of adult T-cell leukemia (ATL) had been described in Japan. ATL is characterized by several clinical features, including skin lesions, hypercalcemia, the presence of leukemic cells with multilobulated nuclei, and an aggressive clinical course.354,376 Patients with ATL were clustered in a limited area of Japan, including the islands of Kyushu and Okinawa, which suggested a pathogen(s) as a causative agent. In 1981, it was reported that ATL patients had antibodies that reacted with ATL cell lines,131 suggesting that ATL was caused by an unknown virus. Thereafter, it became evident that the sequence of the provirus in ATL cells was almost identical to that of HTLV. The clinical features of ATL resemble those of mycosis fungoides. Therefore, the patient from whom the HTLV-infected cell line was established might have been misdiagnosed. The diagnosis should have been ATL.
In 1984, HTLV-1 was reported to be associated with tropical spastic paraparesis (TSP),93 and the same disease was reported in Japan as HTLV-1–associated myelopathy (HAM).281 Both diseases were subsequently classified as the same disease and are referred to as HAM/TSP. Other inflammatory diseases were found to be linked to this virus, including HTLV-1–associated uveitis, myositis, alveolitis, and infective dermatitis.116,204,242,243,258,341
HTLV-2 was discovered in a cell line that was established from a patient with T-cell variant hairy cell leukemia.311 Thereafter, infection with this virus was found in the native Amerindian population of North, Central, and South America, in people in Central and West Africa, and in drug abusers in the United States and Europe.75 HTLV-2 has been reported to cause a neurodegenerative disease resembling HAM/TSP.140 However, no definite associations with lymphoproliferative diseases have been reported.
In addition to HTLV-1 and −2, HTLV-3 and −4 were isolated from bushman hunters in Cameroon.400 There are limited numbers of individuals infected with HTLV-3 and −4; thus, their pathologic roles in human diseases are unclear.
Infectious Agent
HTLV is a delta-type retrovirus, and an HTLV virion contains two copies of single-stranded genomic RNA. Upon viral entry into a host cell, the viral RNA is reverse transcribed (the transfer RNA for proline being utilized as a primer), and the viral genome becomes integrated into the host cellular DNA as a provirus. This genome encodes the structural (Gag, Env), enzymatic (protease, reverse transcriptase, integrase), nonstructural regulatory (Tax, Rex), and accessory proteins (p12, p30, p13, HBZ).
Of these proteins, only HBZ is encoded by the minus strand of the HTLV-1 provirus.
Of these proteins, only HBZ is encoded by the minus strand of the HTLV-1 provirus.
HTLV-1 Gene Regulation
Overall HTLV-1 Messenger RNA Regulation
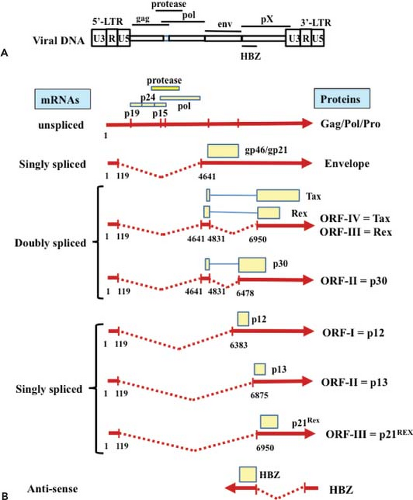
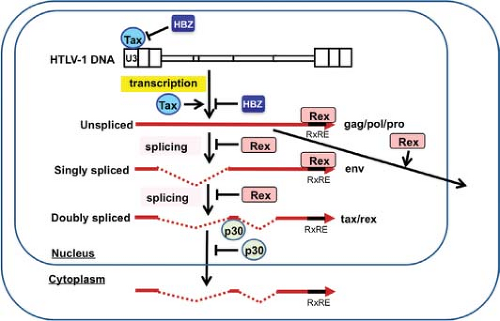
In addition to genomic unspliced messenger RNA (mRNA), HTLV-1 expresses multiple other mRNAs with distinctive splicings314,315 (Figs. 48.1 and 48.2). After infection of host cells, the initial dominant mRNA is the default doubly spliced tax/rex mRNA encoding the x-III and the x-IV open reading frame (ORF). Because the protein product Tax is a transcriptional activator of HTLV-1 transcription, Tax further amplifies the HTLV-1 transcripts, especially tax/rex mRNA, and their protein products. Once the other product, Rex, accumulates in a sufficient amount, it inhibits the nuclear export of tax/rex mRNA, instead increasing the export of singly spliced env mRNA and unspliced genomic RNA encoding gag/pro-pol (see Fig. 48.2), resulting in HTLV-1 release. Thus, Tax and Rex are essential for efficient HTLV-1 replication and production.
Tax-Dependent Transcriptional Activation
Tax is a protein of 353 amino acids, localized in both the nucleus and cytoplasm.101 Tax activates HTLV-1 transcription through the long terminal repeat (LTR)74,269,317,318,339 (see Fig. 48.2). The HTLV-1 LTR is divided into three regions: U3, R, and U5. Three repetitive 21-bp sequences in the U3 region act as Tax-responsive enhancers (TREs).323 Each 21-bp TRE is in turn composed of three elements—the core element and the elements 5′ and the 3′ to the core element—all of which are indispensable for Tax activation of HTLV-1 transcription. The core sequence of a TRE is highly similar to a cyclic adenosine monophosphate (cAMP) response element (CRE) and indeed acts as a binding site for the CRE binding protein (CREB)/activating transcription factor (ATF) family of transcription factors (CREB/ATF).29,421,429,430 Thus, a TRE is also referred to as a viral cAMP response element (vCRE) and is responsive to cAMP. The 5′ and 3′ elements within a TRE are GC-rich. Although Tax does not interact with TRE DNA by itself, it can do so when it interacts with DNA-bound CREB.429,430 When this happens, DNA-bound Tax interacts
with the minor groove of the adjacent GC-rich sequences to stabilize the protein-DNA complex.214,215
with the minor groove of the adjacent GC-rich sequences to stabilize the protein-DNA complex.214,215
CREB is ubiquitously expressed and regulates several cellular genes, especially cAMP-induced genes.398 A cAMP-initiated signal phosphorylates CREB at serine 133, recruiting two transcriptional co-activators, CREB binding protein (CBP) and p300. An in vitro chromatin-based transcription study indicated that phosphorylation of CREB is essential for Tax activity through stabilizing the Tax/CREB/CBP/DNA complex. Moreover, Tax by itself induces the phosphorylation of CREB.191 Consistent with this observation is the fact that a drug that increases the intracellular cAMP level, and thus the phosphorylation of CREB, augments the Tax activation of HTLV-1 transcription.
CBP/p300 is a histone acetyltransferase that acetylates histone tails, thereby changing chromatin structures to activate transcription. In vitro and in vivo studies showed that p300 is crucial for Tax-dependent transcriptional activation, where p300, interacting with Tax and CREB, induces the acetylation of nucleosome histones over the TRE DNA.90,91,99,121,122,202,212,215,269 Interestingly, p300 recruitment by Tax to an integrated HTLV-1 promoter reduces the amount of histone H1 and H3 proteins on the promoter DNA, and stimulates the recruitment of RNA polymerase II.211 These results suggest that recruitment of CBP/p300 to the integrated promoter acetylates histone tails, thereby removing histone octamers from the HTLV-1 promoter. Histone chaperon NAP1 is a factor promoting Tax/CBP/p300-mediated histone eviction.319
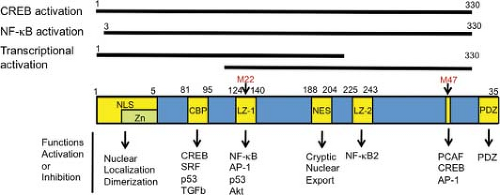
p300/CBP-associated factor (PCAF) is a co-activator for various cellular transcription factors, and it also augments Tax-induced transcriptional activation through the HTLV-1 promoter.166 Like CBP/p300, PCAF is a histone acetyltransferase; however, its activity is dispensable for co-activation of Tax. The well-known Tax mutant M47 (L319R, L320S), which is inactive for transcriptional activation of the HTLV-1 promoter but active for that of NF-κB pathway, is defective in interacting with PCAF (Fig. 48.3).166,338
Treatment of HTLV-1–infected T-cell lines with a histone deacetylase (HDAC) inhibitor (trichostatin A [TSA]) augments the expression of tax/rex mRNA as well as Tax protein, suggesting that HDAC(s) may inhibit HTLV-1 transcription in HTLV-1–infected cells.221 Consistent with this hypothesis, HDAC1 and HDAC3 were found to inhibit Tax-induced transcriptional activation, and this inhibition was rescued by TSA treatment or expression of CBP.70,388 HDAC1 and HDAC3 were shown to interact with Tax, and chromatin immunoprecipitation analysis showed that HDAC1 was associated with integrated HTLV-1 promoter DNA. The amount of promoter-associated HDAC1 was reduced by expression of Tax, indicating that Tax, through interacting with HDAC1, dissociates HDAC1 from the integrated HTLV-1 promoter DNA.
TORCs and P-TEFb (CDK-9/cyclin T1) are additional positive regulators for Tax-dependent HTLV-1 promoter activation.53,54,167,197,336,434 Both proteins interact with Tax, and the knockdown of either by small interfering RNA (siRNA) reduces Tax-dependent activation. On the other hand, Bcl-3, by interacting with TORC3, acts as a negative transcriptional regulator of the HTLV-1 LTR.137
Posttranscriptional Regulation of Viral RNA by Rex
HTLV-1 has two posttranscriptional regulators: Rex and p30.181,423 The roles of Rex and p30 in posttranscriptional mRNA regulations are described later.
In addition to unspliced genomic mRNA encoding gag/pro-pol, HTLV-1 produces singly spliced env mRNA and doubly spliced tax/rex and p30 mRNAs. RNAs with introns generally undergo splicing by the cellular RNA machinery; otherwise, they are degraded. Thus, upon initial infection of host cells, HTLV-1 dominantly expresses doubly spliced tax/rex and p30 mRNA. Once the Rex protein accumulates, Rex controls the ratio of spliced forms of HTLV-1 mRNAs125,150 (see Fig. 48.2). Rex increases the amount of singly spliced (env) and unspliced (gag/pro-pol) mRNAs, and reduces the amount of its own doubly spliced mRNA. Rex does this by inhibiting the splicing of singly spliced (env) and unspliced (gag/pro-pol) mRNAs, stabilizing them, and promoting their transport to the cytoplasm. In the absence of Rex, unspliced HTLV-1 mRNAs are retained in the nucleus owing to two cis-acting repressive sequences (CRS) in the 5′ and 3′ LTR;193,316 however, Rex overcomes the inhibitory activity of the CRS and induces the translocation of the unspliced RNAs into the cytoplasm.
These Rex functions are achieved through at least three activities. The first one is sequence-specific RNA binding. Rex interacts specifically with the HTLV-1 RNA at the Rex-responsive element (RxRE) located in the U3 and R regions of the 3′ LTR.3,40,378 RNA binding by Rex is accomplished by an arginine-rich highly basic region in Rex (aa1–19).114 This domain also contains a nuclear localization signal (NLS) and a nucleolus targeting signal, which allow Rex to enter the nucleolus where it can interact with the RNA.268,335 The RxRE RNA forms a long stem-loop structure, and one stem loop (called stem-loop D) is essential for Rex binding and the function.106 The second Rex function (multimerization) is mediated through aa32–133.396 Rex mutants that cannot form multimers behave as dominant-negative proteins.38,395 The third Rex function is interaction with the nuclear export receptor CRM1/exportin 1, which mediates the transport of viral mRNAs from the nucleus to the cytoplasm. Interaction with CRM1 is also required for multimerization of Rex, because a Rex mutant defective for CRM1 interaction failed to form a multimer. Rex contains a typical leucine-rich nuclear export signal (NES) (aa81–94) that mediates its interaction with CRM1. HTLV-1 production is less efficient in rat cells than human cells because Rex is less active in rat cells than human cells. The rat CRM1 is impaired in promoting Rex to form multimers, which may explain the reduced activity of Rex in rat cells.111,426
Rex is activated by phosphorylation.187 Whereas phorbol myristate acetate (PMA), an activator of protein kinase C (PKC), transiently enhances phosphorylation of Rex, treatment of HTLV-1-infected cell lines with a PKC inhibitor reduces the amount of unspliced gag-pol mRNA.2 Liquid chromatography tandem mass spectrometry analysis showed that Rex is phosphorylated at multiple sites, and mutation analysis indicated that phosphorylations at Ser-97 and Thr-174 are critical for its function.187
Posttranscriptional Regulation of Viral RNA by p30
p30 is encoded by the doubly spliced mRNA of x-II ORF and is a protein of 241 amino acids.21,27 It is unusually rich in Ser (23%) and Arg (12%), and highly basic with theoretical isoelectric point of 11.71. p30 is localized in the nucleus and the nucleolus,26,97,98 and it interacts with the ribosomal protein RPL18a.98
p30 inhibits the nuclear export of doubly spliced tax/rex mRNA through retaining the mRNA in the nucleus.262 p30 binds to the splice junction region (env exon) of tax/rex mRNA. p30 has four NLS and is retained in the nucleus.98 In addition, a fraction of p30 is localized in the nucleolus, although this localization is not essential for its activity. Studies of p30-green fluorescent protein fusion proteins identified two nucleolar retention signals, each consisting of a short Arg-rich stretch. Although p30 has a peptide with homology to an NES, heterokaryon assays indicate that p30 is not a shuttling protein.
Interplay Between Rex and p30
Although both Rex and p30 interact with HTLV-1 RNA and decrease the amount of tax/rex mRNA exported from the
nucleus, they generally have opposite functions in virus production. Whereas Rex stimulates virus production through augmenting the expression of structural proteins (Gag, Pol, Env), p30 has no direct effect on virus production and in fact inhibits it through reducing the expression of Tax and Rex. Interestingly, the Rex and p30 proteins interact with each other, and their interaction is augmented by the presence of the viral mRNAs.333 p30 has little effect on Rex activity; thus, Rex counteracts p30 activity and induces the expression of Tax/Rex proteins. Therefore, the ratio of these two proteins may be a factor controlling the transition between virus latency and virus production.
nucleus, they generally have opposite functions in virus production. Whereas Rex stimulates virus production through augmenting the expression of structural proteins (Gag, Pol, Env), p30 has no direct effect on virus production and in fact inhibits it through reducing the expression of Tax and Rex. Interestingly, the Rex and p30 proteins interact with each other, and their interaction is augmented by the presence of the viral mRNAs.333 p30 has little effect on Rex activity; thus, Rex counteracts p30 activity and induces the expression of Tax/Rex proteins. Therefore, the ratio of these two proteins may be a factor controlling the transition between virus latency and virus production.
Interplay of p30 with HBZ
HBZ is another virally encoded gene (discussed later). Ectopic expression of HBZ RNA increases the expression of tax mRNA and Tax protein from HTLV-1–infected cells. Moreover, HBZ blocks the p30-mediated inhibition of Tax protein expression from tax/rex mRNA.55 This is likely owing to an antisense mechanism, because p30 transcripts originate from a region overlapping with HBZ but in the opposite orientation.
Transcriptional and Posttranscriptional Regulation of Cellular Genes by p30
p30 can play roles in the HTLV-1 life cycle by regulating cellular genes.235,366,424,425 Microarray analysis using total and cytoplasmic mRNA of peripheral blood mononuclear cells (PBMCs) indicated that p30 up-regulates 15 cellular genes and down-regulates 65 at the transcriptional level. In addition, nuclear export assays indicated that p30 up-regulates 33 mRNAs and down-regulates 90 at a posttranscriptional level.366 Human T cells immortalized by a p30-defective HTLV-1 mutant were more sensitive to camptothecin (an anticancer drug)-induced apoptosis than those immortalized by wild-type virus, suggesting that p30 may influence sensitivity to apoptosis. In addition, p30 interacts with CBP/p300 and Tax.424,425 Through this interaction, p30 inhibits Tax as well as CREB-mediated transcriptional activation of the HTLV-1 LTR.
Phenotypes of Rex and p30 Knockout HTLV-1
Knockout studies indicate that Rex is essential for persistent HTLV-1 infection in rabbits but not required for immortalization of human T cells in vitro.417 The defect in infection can be explained by the mRNA export regulatory function of Rex described earlier: Infection cannot be established if virus production is insufficient. On the other hand, studies of a p30-defective HTLV-1 (a stop codon at amino acid position 3 of p30 with an HBZ mutation of Ser-153 to Tyr) suggested that p30 is dispensable for both infectious virus production and immortalization of primary human T cells in vitro and persistent infection in rabbits.332 Nevertheless, it is essential for efficient viral propagation in macaques.381 Twelve weeks after infection of macaques, all of the p30-defective HTLV-1 had reverted to wild-type virus, indicating that p30 was crucial for maintaining HTLV-1 persistent infection. Interestingly, whereas this p30-knockout HTLV-1 produced the viral antigen equivalently to wild-type virus in a B-cell line, it does poorly in human dendritic cells (DCs),381 suggesting that HTLV-1 infection of DCs is involved in persistent HTLV-1 infection in macaques and that p30 is necessary for this function. HTLV-1–infected DCs can transfer HTLV-1 to CD4+ T cells, and this transfer may play a crucial role in the maintenance of persistent HTLV-1 infection.
Latent HTLV-1 Infection In Vivo
Whereas HTLV-1–transformed T-cell lines in vitro express abundant amounts of HTLV-1 mRNA and proteins, fresh PBMCs of HTLV-1–infected individuals generally express only a polymerase chain reaction (PCR)-detectable level of viral mRNA and an undetectable level of viral proteins, indicating that HTLV-1 expression is suppressed in these cells. However, once such HTLV-1–infected PBMCs are cultured in vitro, gene expression is initiated within a few hours.
Reactivation of HTLV-1 Transcription
It should be noted that infected individuals have high titers of antibodies against HTLV-1 and high anti–HTLV-1 cytotoxic T-lymphocyte (CTL) activity. Thus, HTLV-1 replication must occur either transiently but repeatedly or constitutively in specific cells other than peripheral blood lymphocytes, which are latently infected. Indeed, there are several circumstances in which HTLV-1 is reactivated to produce viruses. First, several stress agents induce expression of HTLV-1. For instance, arsenic trioxide, an oxidative stress response inducer, enhanced virus production.11 In addition, deprivation of interleukin 2 (IL-2) from IL-2–dependent HTLV-1-immortalized T cells induced expression of the HTLV-1 gag protein.391 This result suggests that the levels of IL-2 may regulate HTLV-1 gene expression in vivo.
Tax
In addition to activation of viral transcription, Tax plays pivotal roles in HTLV-1 immortalization of T cells, persistent infection, inflammation, and pathogenesis. Such pleiotropic Tax functions are introduced in the following sections.
Tax Plays Crucial Roles in HTLV-1 Immortalization and Persistent Infection
Co-culture of lethally irradiated HTLV-1–infected T cells with PBMCs or cord blood mononuclear cells (CBMCs) in the presence of IL-2 for approximately 10 weeks establishes immortalized HTLV-1–infected T-cell lines (called HTLV-1–immortalized cells), and long-term culture of such cells occasionally confers IL-2–independent growth to some cells (called HTLV-1–transformed cells).241,409 It should be noted that such HTLV-1–immortalized/transformed cells do not produce IL-2. These results suggest that in infected individuals, HTLV-1 establishes lifelong persistent infection by immortalizing/transforming T cells, and these cells grow in an IL-2–dependent and/or an IL-2–independent manner.
Tax is indispensable for immortalization/transformation of human T cells as well as for persistent HTLV-1 infection in a host, because deletion or mutation of the tax gene in recombinant HTLV-1 abrogates immortalization as well as persistent infection in rabbits.300 Moreover, Tax by itself, without other viral products, immortalizes primary human and rat T cells in the presence of IL-2 in vitro.4,104,251 However, transduction of Tax alone into PBMC does not lead to IL-2–independent transformation of T cells, suggesting that HTLV-1 has another factor required for IL-2–independent transformation. The functions of Tax associated with immortalization of T cells and HTLV-1 pathogenesis is introduced in the following section.
Activation of NF-κB by Tax
Tax activates the transcription factor NF-κB, thereby inducing the expression of several cellular genes (Fig. 48.4), and this activity is crucial for multiple aspects of the HTLV-1 life cycle.22,41,168,216,231,301,328,342 For instance, recombinant HTLV-1 carrying a mutant Tax that cannot activate NF-κB fails to immortalize human T cells in vitro.300 Moreover, several NF-κB inhibitors induce prominent apoptosis in HTLV-1–infected T cells, indicating that NF-κB is crucial for survival of HTLV-1–infected T cells.
NF-κB is a family of transcription factors including NF-κB1 (p50), p65, c-Rel, NF-κB2 (p52), RelB, and Bcl-3, and these factors are divided into two groups belonging to the canonical (NF-κB1/p50, p65, c-Rel) and the noncanonical (NF-κB2/p52, RelB, Bcl-3) pathways. These two NF-κB pathways are both activated by Tax (see later) in T cells.403
To activate the canonical pathway, Tax interacts with several NF-κB regulators. IKKγ is essential for Tax activation of NF-κB, because the mutation of IKKγ in fibroblasts or T cells totally abrogates Tax activation of NF-κB.56,57,89,120,411 IKKγ is a scaffold component of the IκB kinase (IKK) complex, IKKα/IKKβ/IKKγ. Through interacting with IKKγ, Tax activates IKKβ to induce phosphorylation and degradation of IκBs (IκBα, IκBβ), thereby allowing nuclear translocation of p50/p65.56,89,102,223,344 In addition, Tax activates IKKα to phosphorylate p65 (at Ser-536), which stimulates its transcriptional activation function.270
Whereas NF-κB in primary T cells is activated transiently by various immune signals such as antigenic stimulation, it is constitutively active in HTLV-1–infected or Tax-expressing cells. Transient activation of NF-κB in normal cells is regulated by several negative feedback regulators. Tax interacts with one such negative regulator, Tax1BP1, via the adaptor optineurin,270,320 inducing constitutive NF-κB activation by Tax.
CBP/p300 appears to play a role in not only the CREB/ATF pathway but also in the NF-κB pathway. Tax and NF-κB/p65 form a ternary complex with CBP in the nucleus of HTLV-1–infected T cells, and this complex is associated with activation of the NF-κB pathway.350 In addition to p65, Tax has been shown to interact with other NF-κB subunits (p50, p52, c-Rel) as well as its inhibitors (IκBα, p105), although the precise roles of these interactions in NF-κB activation by Tax have not yet been established.132,133,249,345 Tax also interacts with MEKK1, TAK1, TAK1-binding protein 2, and protein phosphatase 2A, which are also associated with activation of NF-κB by Tax.19,78,141,402,419
Tax also activates the noncanonical NF-κB pathway.403 Tax1 simultaneously binds to the IKK complex (IKKγ, IKKα) and NF-κB2/p100, but not IKKβ, and thus induces IKKα-mediated phosphorylation of p100, its processing into p52, and the subsequent translocation of p52/RelB into the nucleus. The knockdown of NF-κB2/p100 by short hairpin RNA (shRNA) reduces Tax-induced IL-2–independent growth induction in CTLL-2 cells.128 Thus, the noncanonical NF-κB pathway is also crucial for the transforming activity of Tax.
Apoptosis Inhibition by Tax
HTLV-1–infected T cells are resistant to various types of apoptosis, and this resistance is mostly mediated by Tax.184,248 Tax inhibits apoptosis of T cells induced by IL-2 withdrawal in an IL-2–dependent T-cell line (CTLL-2). Studies of Tax mutants (M47, M22) indicate that inhibition of apoptosis is mediated through the NF-κB pathway.157 Tax activates the expression of several anti-apoptotic genes in T cells, including CTLL-2 cells through the NF-κB pathway. These genes include bcl-xl, xIAP, cIAP, and cFLIP.373 Tax also represses the expression of pro-apoptotic genes such as Bax.43
Activation of the PI3K/AKT Pathway by Tax
IL-2 activates PI3K and its downstream kinase, Akt, in normal T cells—events that are essential for apoptosis inhibition and cell growth. However, even in the absence of IL-2, HTLV-1–transformed T-cell lines show constitutive activation of the PI3K/Akt pathway. Moreover, several inhibitors of this pathway, such as LY294002 (an inhibitor for PI3K), induce growth arrest of HTLV-1–transformed T-cell lines and then induce apoptosis, suggesting that the ongoing activation of this pathway is essential for maintaining survival of HTLV-1–infected T cells.146,162,163 To do this, Tax directly interacts with PI3K, which consists of two subunits: the catalytic p110α subunit and the inhibitory p85α subunit. Tax directly binds to
the p85α inhibitory subunit, causing the release of the active p110α catalytic subunit.287
the p85α inhibitory subunit, causing the release of the active p110α catalytic subunit.287
Activation of the PI3K/Akt pathway by Tax results in activation of the downstream target mammalian target of rapamycin (mTOR) in HTLV-1–infected T cells as well as Tax-expressing cells.422 Rapamycin, an inhibitor of mTOR kinase, inhibits the growth of HTLV-1–transformed T cells and induces G1 cell cycle arrest.146 This growth inhibition of HTLV-1–infected T cells is associated with reduced phosphorylations of p70S6 kinase and 4E-BP1.
G1-S Promotion by Tax
In the absence of IL-2, ectopic expression of Tax in an IL-2–dependent human T-cell line (Kit225) or in PHA-activated PBMC induces cell cycle progression from G0/G1 into S phase.261,273,312 This activity is associated with the activation of cyclin-dependent kinase 2 (CDK2) and CDK4. CDK2 and CDK4 phosphorylate a family of retinoblastoma (Rb) proteins. The phosphorylation of Rbs induces the release of the transcription factor E2F, freeing E2F to activate the expression of its target genes that control the cell cycle.
Tax has multiple distinct ways of stimulating cell cycle progression. First, Tax was found to activate CDK4 by interacting directly with p16INK4a, a CDK4 inhibitor.346 However, a subsequent study showed that Tax stimulates cell cycle progression even in a p16INK4a-defective T-cell line.213 Indeed, Tax can also interact directly with CDK4 and its inhibitor p15INK4b, stimulating the kinase activity.113,220,347 Studies of Tax mutants (M22, M47) indicate that in IL-2–dependent Kit225 cells, E2F activation by Tax occurs via the NF-κB pathway.273 E2F is a family of proteins (E2F1–E2F6). Whereas expression of E2F1 in a G0/G1-arrested fibroblast cell line induces cell cycle progression into S phase, it does not so in PHA-stimulated PBMC.273 Because Tax stimulates cell cycle progression in PHA-stimulated PBMC, in addition to E2F1 activation, Tax must have another activity that stimulates cell cycle progression in PBMC. Indeed, Tax induces the transcription of several genes associated with cell cycle regulation, including cyclin D1, cyclin D2, cyclin E, CDK2, CDK4, CDK6, and E2F1, and represses the transcription of the cell cycle inhibitor p18INK4c.5,155,273,305,347 In addition, knockdown experiments using short-hairpin RNA show that cyclin D2, CDK6, NF-κB/p65, and NF-κB2/p100 all play a role in E2F activation as well as cell cycle progression induced by Tax in Kit225 cells.156 Collectively, these results suggest that Tax stimulates cell cycle progression through multiple mechanisms.
Genomic Instability in HTLV-1–Infected T Cells
HTLV-1 infected T-cells, including leukemic cells from ATL patients, have numerous genetic abnormalities such as DNA mutations, chromosomal translocations, deletions and duplications, and aneuploidy, and these genetic aberrations are linked to ATL development. For examples, homozygous deletions of p16 (CDKN2A) and p15 (CDKN2B) genes (tumor suppressor genes that regulate the cell cycle) were frequently observed in aggressive acute and lymphoma-type ATL.124 The major viral factor that induces such genomic abnormalities is Tax, which acts via several distinct mechanisms as described later.
P53 Inactivation by Tax
The tumor suppressor p53 is the “guardian of the genome” in DNA damage responses. Under normal conditions, p53 is functionally inactive; however, upon the incidence of almost any stress, including DNA damage, p53 is accumulated, stabilized, and activated to gain competence in the transcriptional activation of genes that contribute to cell cycle arrest, apoptosis, and senescence. In HTLV-1-infected cells, such p53 functions are inactivated by Tax.6,248,295,412 Tax interacts with the p53 co-activator CBP/p300 and blocks its access to p53, thereby reducing the transcriptional activation function of p53.348 In addition, Tax induces the phosphorylation of p53 at Ser-15—a modification that triggers p53 to form an inactive ternary complex with Tax and NF-κB/p65.164,295 Tax also inactivates two other p53-like proteins (p73 and p63/p51) in an HTLV-transformed T-cell line (MT-2).177,210 Ectopic expression of Tax in p53-negative Saos2 cells inhibits transcriptional activation of p73 and p63/p51 through the N-terminal domains. These results suggest that the inactivation of p53 and p53-related proteins is involved in the genomic instability of HTLV-1–infected cells.
Activation of Telomerase by Tax
Telomeres, the terminal sequences of chromosomes, play a crucial role in preventing chromosome abnormalities from occurring at each cell division; however, telomeres are shortened by every cell division in normal cells. In normal cells, p53-dependent replicative senescence is thus induced after a limited number of cell divisions. On the other hand, HTLV-1–immortalized cells have tricks to escape this telomere shortening, thereby avoiding replicative senescence. In HTLV-1–infected T cells, Tax activates the transcription of human telomerase reverse transcriptase (hTERT), an enzyme that synthesizes telomeric sequences and thereby extends telomeres.118,334 Studies using Tax mutants (G148V, M22) defective in NF-κB activation and chromatin immunoprecipitation analysis showed that Tax induces increased binding of Sp1 and c-Myc to the hTERT promoter through the NF-κB pathway.334
IL-2–dependent HTLV-1–immortalized T cells generally express a low amount of Tax—an amount of which may not be sufficient to maintain telomerase activity. However, IL-2 augments telomerase activity in HTLV-1–immortalized T cells. IL-2 signals inhibit the nuclear translocation of the Wilms tumor protein, an inhibitor of hTERT transcription, through a PI3K-dependent but Akt-independent pathway.31 The IL-2 dependence of telomerase activity in HTLV-1–infected cells suggests that continued growth of such cells in low IL-2 conditions may lead to shortened telomeres and chromosomal aberrations.
Centrosome Amplification
The centrosome is a microtubule organizing center during cell division and plays a crucial role in the precise duplication and segregation of chromosomes. Whereas normal T cells have one centrosome during interphase, in HTLV-1–infected cell lines, around 30% of the cell population has more than two centrosomes, indicating that a centrosomal aberration may contribute to the chromosomal abnormalities observed in HTLV-1–infected cells.52,266 Such abnormal centrosomes are induced by the ectopic expression of Tax in human T cells.
This activity of Tax is mediated through its interaction with several host proteins such as TAX1BP2 and RanBP1.52,266 Endogenous TAX1BP2 is localized in the centrosome, and its knockdown induces centrosome amplification. Furthermore,
overexpression of TAX1BP2 reduces Tax-induced centrosome amplification. Collectively, these results indicate that Tax induces centrosome aberrations by inactivating TAX1BP2. In contrast, the knockdown of RanBP1 abrogates centrosome amplification by Tax as well as centrosome localization of Tax, indicating that RanBP1 targets Tax to centrosomes. MAD1 is another Tax-interacting protein localized in the centrosome during metaphase. Whereas the knockdown of MAD1 induces multinucleated cells, its overexpression inhibits induction of multinuclear cells by Tax.169 Thus, MAD1 is also involved in the genomic instability induced by Tax in HTLV-1–infected cells.
overexpression of TAX1BP2 reduces Tax-induced centrosome amplification. Collectively, these results indicate that Tax induces centrosome aberrations by inactivating TAX1BP2. In contrast, the knockdown of RanBP1 abrogates centrosome amplification by Tax as well as centrosome localization of Tax, indicating that RanBP1 targets Tax to centrosomes. MAD1 is another Tax-interacting protein localized in the centrosome during metaphase. Whereas the knockdown of MAD1 induces multinucleated cells, its overexpression inhibits induction of multinuclear cells by Tax.169 Thus, MAD1 is also involved in the genomic instability induced by Tax in HTLV-1–infected cells.
Defective DNA Repair
DNA repair activity is attenuated by Tax in HTLV-1–infected T-cell lines.160,179,227,238,290 Ataxia telangiectasia mutated (ATM), a member of the PI3K-like kinase family, is activated by various DNA damaging agents, and its activation is essential in initiating signaling cascades that stop DNA replication and allow the repair of damaged DNA. ATM activation by ionizing radiation (IR), and signals downstream of ATM activation, are diminished in HTLV-1–infected T-cell lines as well as cells expressing Tax alone.49 Upon treatment with IR, both normal and Tax-expressing cells stop DNA replication; however, the interval before replication resumes is shorter in Tax-expressing cells than in the parental control cells; simultaneously, the cells expressing Tax are defective for DNA repair activity. Although the precise mechanism by which Tax blocks DNA repair has not yet been fully elucidated, Tax is known to interact with several host factors involved in DNA repair, such as DNA-dependent protein kinase, Ku, Chk1, and Chk2.68,284,285 In addition, Tax represses transcription of the DNA polymerase β gene, which plays a crucial role in DNA repair.160
Cell Cycle Arrest Induced by Tax
Surprisingly, under certain experimental conditions, overexpression of Tax induces cell cycle arrest at G1 or G2-M as well as senescence. For instance, ectopic expression of Tax in HeLa cells or an IL-2–independent human T-cell line (supT1) induced G1 cell cycle arrest. Moreover, transient HTLV-1 infection of HeLa or SupT1 cells also induced G1 cell cycle arrest. These arrests are correlated with the Tax-dependent induction of CDK2 inhibitors (p21/waf1 and p27Kip1). In contrast, several established IL-2–independent HTLV-1–transformed T-cell lines express a high amount of p21 without cell cycle arrest, suggesting that during in vitro culture, HTLV-1–transformed cell lines may gain genetic change(s) that overcome cell cycle arrest. Based on these observations, it has been proposed that HTLV-1–infected cells in vivo are selected for their ability to escape such antigrowth effects of Tax, accumulating genetic alteration(s) during long-term HTLV-1 infection, and that this phenomenon may play a role in the numerous genetic alterations observed in HTLV-1–infected cells in vivo, such as chromosomal abnormalities. However, it should be noted that Tax also inactivates the growth-inhibitory functions of p21 through activating Akt: It has been reported that the activation of Akt in HTLV-1–transformed T-cell lines induces phosphorylation of p21, thereby inducing its translocation into the cytoplasm, where it is inactivated.392
Reactive Oxygen Species Induction by Tax
Ectopic expression of Tax in T cells stimulates the production of reactive oxygen species (ROS), which induce DNA damage (detected by a comet assay and γH2AX staining) and senescence (detected by β-galactosidase staining).194 A ROS scavenger (N-acetylcysteine) abrogates DNA damage induced by Tax, indicating that ROS mediate the DNA damage induced by Tax. These results indicate that ROS may also contribute to the genomic abnormalities observed in Tax-expressing cells.
Inflammation Induced by Tax
Increased Productions of Cytokines and Chemokines in HTLV-1–Infected Cells
One prominent feature of HTLV-1–infected T cells is the production of several cytokines and chemokines, as well as expression of their receptors. These gene inductions are mostly mediated by Tax through the NF-κB, CREB, and AP-1 pathways13,20,190,250,389 and are thought to play pivotal roles in the phenotypes, activation, proliferation, differentiation, and behavior of HTLV-1–infected cells and thereby in HTLV-1 pathogenesis. For example, induction of the interleukin 2 receptor (IL-2R) complex by Tax is crucial for the immortalization of human T cells by HTLV-1.60,149,231 In addition, HTLV-1–infected T cells show prominent adhesion capacity, both homophilic and heterophilic, as well as cell migration activity, and they express several adhesion molecules, chemokines, and chemokine receptors involved in these functions, such as CD40, ICAM-1, OX40, and OX40L—many of which are induced by Tax in T cells.126,147,274,352,361 These properties would promote the resistance of HTLV-1–infected T cells to various pro-apoptotic stimuli, their migration to distant tissues through blood and lymphatic vessels, and their infiltration of tissues. Indeed, the CXCR4 antagonist AMD3100 inhibits the migration of cultured lymphoid cells from either Tax-transgenic mice or ATL patients.183
Activation of AP-1–Dependent Gene Expression by Tax
AP-1 activation by Tax is implicated in cell proliferation induced by Tax.287 The transcription factor AP-1 is transiently activated in normal T cells during antigenic stimulation, and it in turn activates several cellular genes involved in T-cell functions such as prevention of apoptosis, cytokine production, proliferation, and cell migration. In contrast, HTLV-1–infected T-cell lines have constitutive AP-1 DNA binding activity in the nucleus.79 Such constitutive AP-1 activity is mediated by Tax. Through AP-1, Tax has been shown to activate many genes, including IL-5, IL-8, transforming growth factor beta (TGF-β), fra-1, proenkephalin, TR3/nur77, and TIMP-1.50,372,375,407
AP-1 consists of protein dimers. The constituent proteins belong to two groups of transcription factors—the Fos (c-Fos, FosB, Fra-1 and Fra-2) and Jun (c-Jun, JunB, and JunD) families—and AP-1 consists of either homodimers of the Jun family or heterodimers between two groups. Among these factors, c-fos, fra-1, c-jun, and junD are all transcriptionally activated by Tax.79,80,255,372 Analysis of the c-fos promoter identified a CArG box as a Tax-inducible enhancer, where Tax interacts both with the CArG box binding protein serum response factor (SRF) and with ternary complex factor (TCF; Elk-1, SAP-1).81,327,343 In addition, two co-activators, CBP/p300 and P/CAF, are involved in Tax-dependent activation through the CArG box.327 The CArG box acts generally as an inducible enhancer for immediate early genes, such as c-fos, which are induced without de novo protein synthesis. Indeed, Tax, through its
action on the CArG box, induces the expression of various immediate early genes such as c-fos, egr-1, and egr-2.8,79,81
action on the CArG box, induces the expression of various immediate early genes such as c-fos, egr-1, and egr-2.8,79,81
Inhibition of Transforming Growth Factor Beta Signaling by Tax
TGF-β is a cytokine that controls multiple cellular functions, such as cell growth inhibition, differentiation, senescence, and apoptosis, and acts as a tumor suppressor protein in solid tumors as well as leukemia/lymphoma. HTLV-1–infected T-cell lines are resistant to TGF-β–induced growth inhibition, and Tax is responsible for this resistance.16,208,244 TGF-β signaling is mediated through a family of transcription factors referred to as Smads. Tax, through JNK activation, induces phosphorylation of c-Jun, and phosphorylated c-Jun forms an inactive complex with Smad3.16 In addition, Tax blocks the interaction of Smads with the transcriptional co-activator p300.244
Deregulated microRNA in HTLV-1–Infected Cells
Although cellular microRNAs (miRNAs) are a crucial player in the host defense mechanism for clearing viral infections, many viruses have functions that counteract the antiviral activities of miRNAs. In some cases, viruses even utilize miRNAs to maintain persistent infection.302 In HTLV-1–infected cells, several miRNAs are up-regulated or down-regulated relative to those in uninfected T cells.302 Whereas up-regulated cellular miRNAs in HTLV-1–infected cells include miR-21, −24, −93, −130b, −146, −155, and −142–3p, down-regulated ones include miR-125a, −132, −181a, −150, and −223.30,291 Two miRNAs up-regulated in HTLV-1–infected T cells (miR-93 and miR-130b) target the same gene, TP53INP1, which is a p53-inducible tumor suppressor gene.418 Knockdown of miR-93 and miR-130b by antagomirs in either an HTLV-1–transformed T-cell line (MT-4) or the Jurkat T-cell line increases the expression of TP53INP1 and reduces cell viability. Moreover, Tax stimulates the transcription of miR-130b. Collectively, these results suggest that Tax promotes the growth of HTLV-1–infected cells through miR-130b and TP53INP1, and that the up-regulation of certain miRNAs plays a role in the proliferation of HTLV-1–infected T cells, and thereby persistent infection and leukemogenesis.
Several miRNAs deregulated in HTLV-1–infected T cells and uncultured leukemic cells of ATL patients (miR-181a, −142, −223, and −150) are involved in hematopoiesis.30 For instance, miR-181a, a gene down-regulated in HTLV-1–infected cells, is preferentially expressed in the B-lymphoid lineage in mice. Moreover, its ectopic expression in hematopoietic progenitor cells followed by in vitro differentiation increases the yield of the B-lymphoid lineage cells but not T-lymphoid ones. Two miRNAs (miR-150 and miR-223) are overexpressed in the PBMC of ATL patients but down-regulated in HTLV-1–transformed T-cell lines, suggesting that ATL cells and in vitro HTLV-1–transformed T-cell lines may originate from distinct populations. This observation may support the cancer stem cell theory for ATL development discussed later.
Distinct Activities of Tax1 Versus Tax2
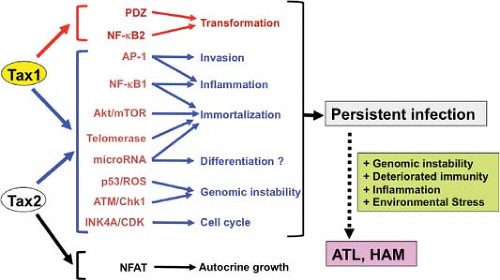
HTLV-1 Tax (Tax1) and HTLV-2 Tax2 have around 80% similarity at the amino acid level and carry out many activities with equal potency. However, there are two important distinctions between the two proteins: Tax1 is associated with greater transforming activity, whereas Tax2 induces IL-2 production more strongly.
The High Transforming Activity of Tax1 Relative to TAX2
CTLL-2 is a mouse cytotoxic T-cell line, the growth of which is strictly dependent on IL-2. The lentivirus-mediated transduction of Tax1 into CTLL-2 transforms these cells, converting them from IL-2–dependent growth to IL-2–independent growth, and surprisingly, the transforming activity of Tax1 is much more potent than that of Tax2.128,371 These results present a simple but attractive hypothesis of the HTLV-1 pathogenesis presented later.127 Whereas HTLV-2–infected T cells could preferentially grow and survive in conditions and/or tissues with relatively high amounts of IL-2, under low IL-2 conditions, HTLV-1–infected T cells could grow more efficiently than HTLV-2–infected cells. This distinctive characteristic of HTLV-1–infected cells would allow more clonal proliferation of infected cells in vivo, which is a prerequisite for ATL development. This observation may explain why HTLV-1, but not HTLV-2, is associated with ATL.
What makes Tax1 more potent than Tax2? The CTLL-2 assay identified at least two Tax1-specific functions responsible for its high transforming activity relative to Tax2: One is its interaction with selective PDZ domain proteins, and the other is its activation of the noncanonical NF-κB2 pathway.128,371
Tax1 Interaction with PDZ Domain Proteins. As mentioned earlier, studies of Tax1-Tax2 chimeras identified a PDZ domain binding motif (PBM) at the C-terminus of Tax1 (missing in Tax2) that is responsible for Tax1’s high transforming activity in CTLL-2 cells. The significance of the Tax1 PBM in HTLV-1 was also indicated by the observation that deletion of the PBM from HTLV-1 (HTLV-1/ΔPBM) abrogates its ability to persistently infect rabbits.405 Whereas the PBM is crucial for IL-2–independent growth of CTLL-2 cells, the PBM is dispensable for IL-2–dependent immortalization of primary human T cells in vitro, supporting the idea that the Tax1 PBM plays a role at low IL-2 conditions but not at high IL-2 conditions.
PBMs are generally located at the carboxy-terminus of cellular and viral proteins, and mediate binding with cellular PDZ (PSD-95/Discs Large/ZO-1) domain proteins. Intriguingly, PBM motifs in transforming viruses are selectively present in oncogenic viruses but not their nononcogenic relatives. For instance, human papillomaviruses (HPVs) have high-risk subtypes such as HPV16 and −18 that are associated with HPV-associated malignancies such as cervical carcinoma and low-risk subtypes that are associated only with benign hyperplastic diseases. The E6 oncoproteins of high-risk HPVs, but not low-risk ones, possess PBMs. A similar association of a PBM with a viral subtype-specific oncogenesis is also seen for human adenovirus type 9, which causes mammary tumors in rats. Interestingly, the PBMs from the oncogenic HPV E6 and the adenovirus E4-ORF1 can substitute for the Tax1 PBM in allowing Tax1 to transform CTLL-2 cells. This observation suggests that the functions of these viral PBMs and the PDZ domain proteins with which they interact are identical, and that these three oncogenic viruses utilize a common mechanism to induce malignancies. PDZ domain proteins that interact with Tax1 include Dlg1, Scribble, MAGI-3, TIP-1, and IL-16 precursor protein.17,271,275,399 Knockdown of Dlg1 increases the
transforming activity of Tax1 in CTLL-2 cells, suggesting that Dlg1 negatively regulates the transforming activity of Tax1.
transforming activity of Tax1 in CTLL-2 cells, suggesting that Dlg1 negatively regulates the transforming activity of Tax1.
Activation of NF-κB2 by Tax1. Unlike Tax1, Tax2 does not activate the noncanonical NF-κB pathway. The major defect of Tax2 here is that Tax2 cannot interact with NF-κB2/p100.128 Knockdown of NF-κB2/p100 prominently reduces the transforming activity of Tax1 in CTLL-2 cells, indicating that Tax1-specific activation of the noncanonical NFκB pathway is crucial for the transforming activity of Tax1. In addition, transduction of p100 siRNA into large granular lymphoma cell lines derived from Tax1-transgenic mice augmented their sensitivities to pro-apoptotic stimuli and simultaneously reduced the expression of anti-apoptotic XIAP and cIAP. The leucine zipper-like region 2 (LZ-2) of Tax1 (aa 225–232) is a crucial region for Tax1-induced NF-κB2 activation (see Fig. 48.3).326
Activation of the Nuclear Factor of Activated T-Cell Pathway by Tax2
Whereas Tax1 has several dominant functions over Tax2, Tax2 has one dominant function over Tax1. Ectopic expression of Tax2 in a human T-cell line (Jurkat) induces the expression of IL-2, and the activity of Tax2 is much more potent than that of Tax1 in this respect.264 Similarly, HTLV-2–infected T-cell lines, but not HTLV-1–infected ones, constitutively produce IL-2 protein in the culture supernatant. Importantly, anti–IL-2R antibodies inhibit the proliferation of HTLV-2–infected T-cell lines, suggesting that the IL-2/IL-2R autocrine loop is essential for this proliferation. IL-2 gene induction by Tax2 in HTLV-2–immortalized T cells is mediated through the nuclear factor of activated T cells (NFATs), especially the NFATp component. In normal T cells, NFAT is retained in the cytoplasm in an inactive, phosphorylated form, and its dephosphorylation (by the phosphatase calcineurin) induces its translocation into the nucleus and allows it to activate transcription of its target genes. Cyclosporine A, an inhibitor of calcineurin, abrogates IL-2 expression induced by Tax2, indicating that Tax2 activates IL-2 production by targeting calcineurin or elements upstream of it.
Tax3 and Tax4
HTLV-3 and HTLV-4 encode Tax3 and Tax4, respectively. Amino acid comparison of these Tax proteins indicates that Tax3, but not Tax4, has a PBM, and Tax3 has been shown to bind to the PDZ domain protein Dlg4.51 On the other hand, in the LZ-2 region (essential for NF-κB2 activation by Tax1), Tax3 and Tax4 show more homology to Tax2 than to Tax1 (see Fig. 48.3). Therefore, these four HTLVs are classified into at least three distinct groups based on the PBM and the LZ-2 region.
In Vitro Immortalizing and Transforming Activities of Tax1
Tax immortalizes primary human or rat T cells in the presence of IL-2. Analysis of Tax mutants (M22, M47) indicates that the NF-κB pathway is crucial for immortalization of T cells. When tax gene is transduced into PBMCs by a retroviral vector, Tax preferentially immortalizes CD4+ T cells. Interestingly, a Tax mutant defective in activating the CREB pathway (M47) preferentially immortalizes CD8+ cells but not CD4+ cells. These results have been confirmed by studies using whole virus: an HTLV-1 mutant carrying the same Tax mutation (M47, M22). These results suggest that the NF-κB pathway plays a major role in immortalization of T cells by Tax, whereas the CREB pathway is involved in the preferential immortalization of CD4+ cells.300
Tax Activities in Animal Models
Tax-transgenic animals (mice or rats) develop various diseases such as CD4+ T-cell leukemia/lymphoma, natural killer (NK) cell lymphoma, thymoma, and neurofibroma.107,123,260,272 For instance, mice transgenic for tax regulated by the mouse lymphocyte-specific tyrosine kinase (Lck) proximal or distal promoter both developed T-cell leukemia/lymphoma: the former, a pre–T-cell lymphoma/leukemia with a double negative (CD4−CD8−) phenotype, and the latter, a mature single-positive (CD4+ or CD8+) T-cell leukemia/lymphoma.123,272 Intriguingly, cancer stem cells (CSCs) were identified from the Lck (proximal)-tax-transgenic mice in a minor population of CD38−/CD71−/CD117+ cells by inoculating the splenic lymphomatous cells into immune-deficient (NOD/SCID) mice.413 Such CSCs originated from pro–T cells or early hematopoietic progenitor cells. Inoculation of HTLV-1–infected or Tax1-transduced human CD34+ hematopoietic stem/progenitor cells (HPS/PC) into humanized SCID mice results in the development of CD4+ T-cell lymphoma.23 These results suggest that HTLV-1 infection of CD34+ HPS/PC may play a role in ATL development.
NF-κB activation plays a crucial role in the oncogenic activity of Tax in animals. For instance, the Tax-transgenic mice developing NK cell lymphoma show constitutive activation of NF-κB, and the inhibition of NF-κB by anti–NF-κB therapeutics perturbs the progression of the disease.236
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree