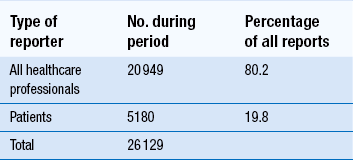
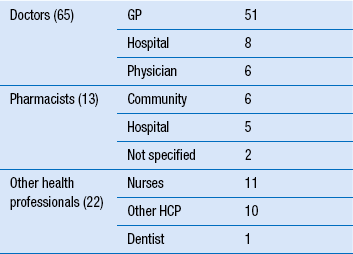
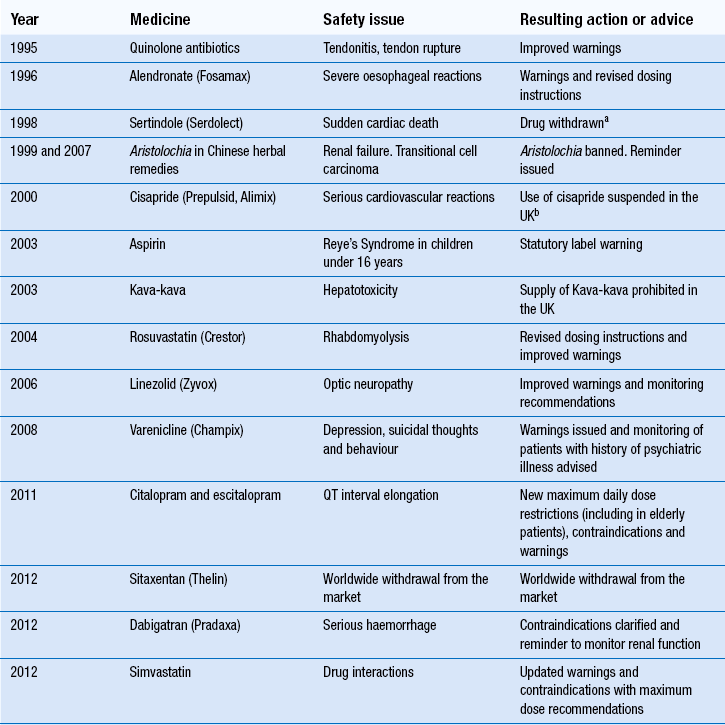
51 Monitoring the patient may conjure up visions of healthcare professionals, including pharmacists, taking samples of blood from the patient, recording measurements or merely observing them in order to manage a medical condition. While these are important examples of how monitoring may be achieved, there are other schemes that are used specifically in relation to monitoring patients and their medication. The Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency which is responsible for assessing the safety, quality and efficacy of a wide range of materials from medicines and medical devices to blood and therapeutic products that are derived from tissue engineering. The MHRA authorizes and regulates their sale or supply for human use in the UK (see Ch. 4). The YCS was introduced in 1964 initially to provide doctors or dentists with a route to report a suspicion that a medicine could have harmed a patient. The YCS gets its name from the colour of the original document used for reporting the ADR. These ‘cards’ have become increasingly more accessible and can be sourced by a variety of methods (Box 51.1). There is also a Yellow card freephone line. The fundamental principles of the scheme have not changed. Proof of a causal link between a medicine or a combination of medicines does not need to be established, so the reports are suspected ADRs. In the UK, the MHRA collates data on ADRs via the YCS from a wide range of healthcare professionals working in the NHS or from private healthcare providers. These now include doctors, dentists, pharmacists (from 1997), nurses, midwives and health visitors (from 2002) and also HM Coroners. Reports are received directly from them and via pharmaceutical companies. The scheme is voluntary for healthcare professionals (HCPs) but pharmaceutical companies holding marketing authorizations have a statutory obligation to report ADRs to the MHRA. Following pilot schemes in 2005–2006, direct reports from patients, parents and carers are now accepted. The Yellow Card report forms for patients are different from those for healthcare professionals. Standard report forms are shown in Figures 51.1 and 51.2. Figure 51.1 Standard Yellow Card report form for reporting suspected adverse drug reactions by healthcare professionals. (Crown Copyright 2012). Figure 51.2 Standard Yellow Card report form for reporting suspected adverse drug reactions by patients, parents and carers. (Crown Copyright 2010). There are a number of problems associated with the YCS but the major one has been, and continues to be, under-reporting. An internet electronic Yellow Card is available (http://www.mhra.gov.uk) to enable all reporters to submit ADR reports via the MHRA website in a paperless way. The online reporting forms have been designed to improve clarity and usability by incorporating ‘drop-down’ menus and dictionaries for technical terms. They allow a reporter to save a partially completed report at any point so that it can be finished and submitted at a convenient time or later when more information had been received concerning the ADR. This method is being strongly promoted to encourage spontaneous reporting. The MHRA and CHM also have five Yellow Card centres whose role focusses on increasing awareness of ADRs and the YCS and follow-up of reports in their areas as this has been shown to improve the number and quality of reports. In the period 1 November 2001 to 31 December 2006, reporting of ADRs initially increased by approximately 5% each year compared with the same period in the previous year, but numbers have remained static, at approximately 25 000 reports per year since then. In a 2-year study period between 1 October 2005 and 30 September 2007, the YCS received 26 129 reports from all patients and HCPs. Table 51.1 provides details on the specialty of the reporters for reports received during this period. Patient reporting has been supported by the distribution of improved Yellow Cards to all GP surgeries, community pharmacies and other NHS outlets across the UK and the freephone line. The patient electronic Yellow Card has been updated and can also be accessed through the website. The study showed that patients had adopted paper, internet and telephone methods of reporting, whereas HCPs appeared to prefer to submit paper reports. However, this may have changed in the interim. All data are closely scrutinized by the MHRA/CHM to determine whether a potential health threat is emerging and further investigation is required or more immediate action needs to be taken. In some cases, marketing authorization for the drug is withdrawn by the regulatory authority when the risks are considered to outweigh the benefits or the company may voluntarily suspend or withdraw the product. In several instances, a drug has continued to be available following amendments to the summary of product characteristics (SPC) and patient information leaflet (PIL) indicating restrictions in use, reduction in dosages and special warnings and precautions. Some examples can be seen in Table 51.2. Complete listings of the suspected ADRs reported to the MHRA through the YCS by HCPs and patients are provided in drug analysis prints (DAPs). Drug analysis prints can be accessed from the MHRA website. Table 51.2 Safety issues which Yellow Card reports have helped to identify aSertindole was reinstated in 2002 with increased warnings.
Monitoring the patient
Introduction
The yellow card scheme




 A different formulation for an existing route
A different formulation for an existing route
 A new combination of active substances
A new combination of active substances
 Administration by a new route which is significantly different from existing routes
Administration by a new route which is significantly different from existing routes
 A change or addition of a therapeutic indication that
A change or addition of a therapeutic indication that
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree