The other commonly used term for adenosis is atypical adenomatous hyperplasia (AAH). We prefer the term adenosis, as prefacing adenomatous hyperplasia with atypical has adverse consequences both in terms of practical patient management and in our theoretical framework of this entity. As outlined in the following text, there are very little data in support of a relation between adenosis and carcinoma. By designating these lesions as atypical, many patients will be subjected to unnecessary repeat biopsies. Conceptually, as has happened in the past, use of the term atypical adenomatous hyperplasia will result in this entity being considered with prostatic intraepithelial neoplasia (PIN) as precursors to carcinoma of the prostate. Whereas there is strong evidence that PIN is a precursor to some prostate cancers, this evidence is lacking in adenosis.
There is a wide spectrum in the literature in terms of the reported incidence of adenosis on transurethral resection of the prostate (TURP), ranging from 2.2% to 19.6%.8 The reason for this broad range is different thresholds for diagnosing a focus of crowded glands as adenosis. Included within the lower threshold are prostate specimens with foci of crowded glands, which could be considered a minimal example of adenosis, although they do not closely mimic adenocarcinoma. Crowded benign glands that have absent or patchy staining for basal cell markers and/or positive racemase immunoreactivity are one of the more frequent mimickers of prostate cancer.9 At the other extreme, seen in 1.6% of benign TURPs performed at The Johns Hopkins Hospital, adenosis closely mimics adenocarcinoma of the prostate. The diagnosis of adenosis should be restricted to cases with a sufficiently atypical growth pattern that one has to seriously consider the diagnosis of low-grade cancer. This gradual spectrum within adenosis from a crowded focus of obviously benign glands to lesions that share similar features, yet more closely resemble cancer, supports the concept that adenosis is a hyperplastic rather than neoplastic lesion. Because adenosis preferentially occurs within the transition zone, it is more frequently seen on TURP as an incidental finding than on needle biopsy. However, in approximately 0.8% of needle biopsies, adenosis may be identified. This incidence is infrequent enough that many pathologists do not consider it in the differential diagnosis of small glandular lesions on needle biopsy. However, the frequency of adenosis on needle biopsy is sufficiently high that there is a good chance that one will see this lesion in one’s practice with the potential to overdiagnose it as adenocarcinoma.
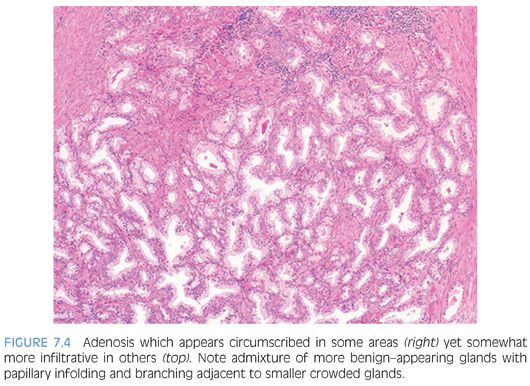
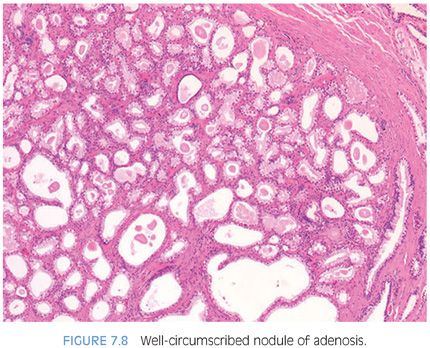
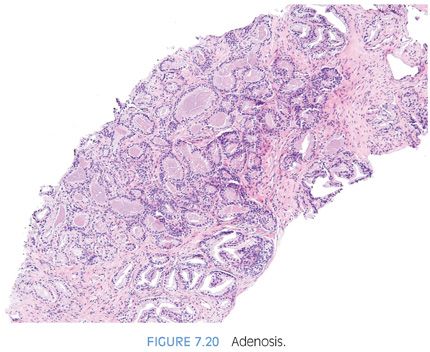
The distinction of adenosis from low-grade adenocarcinoma is based on architectural and cytologic features (Table 7.2). In order to minimize misdiagnoses, the constellation of histologic features seen in a lesion should outweigh the significance of any one diagnostic feature (eFigs. 7.1 to 7.115). At scanning magnification, adenosis is characterized by a lobular proliferation of small glands (Figs. 7.1 to 7.5). In contrast, low-grade carcinoma has a haphazard, irregular, infiltrative growth pattern. Despite the overall lobular pattern seen in adenosis, 19% of cases reveal minimal infiltration of glands into the surrounding stroma (Fig. 7.4).
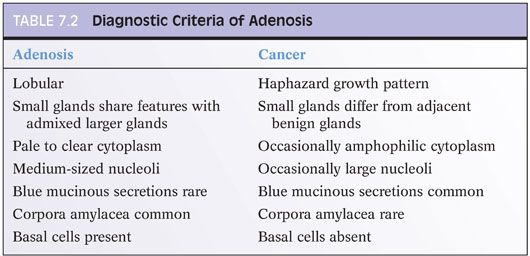
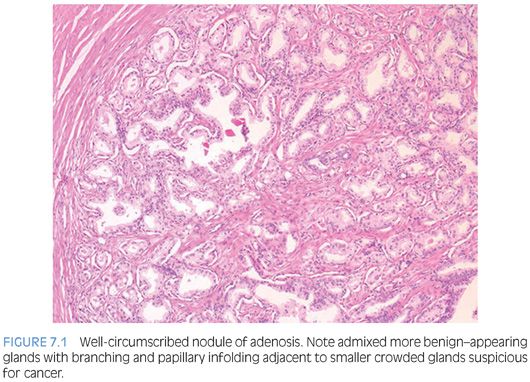
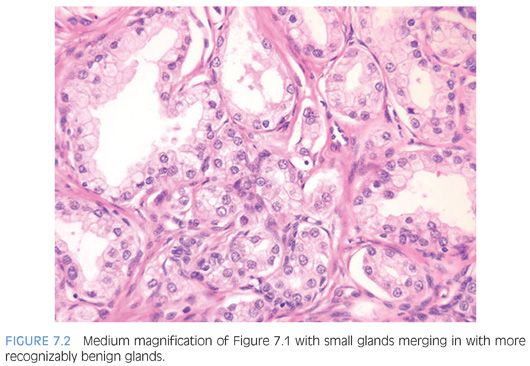
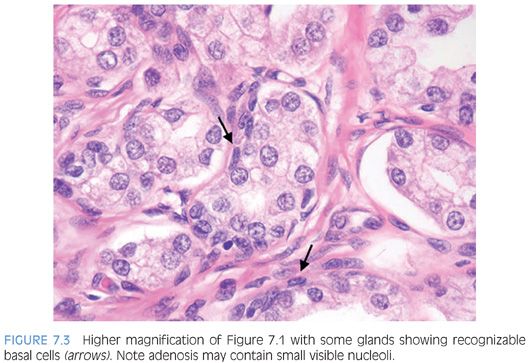
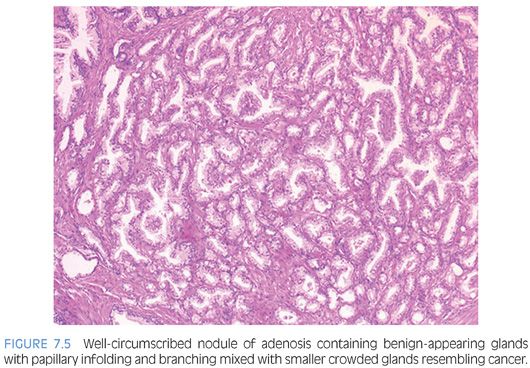
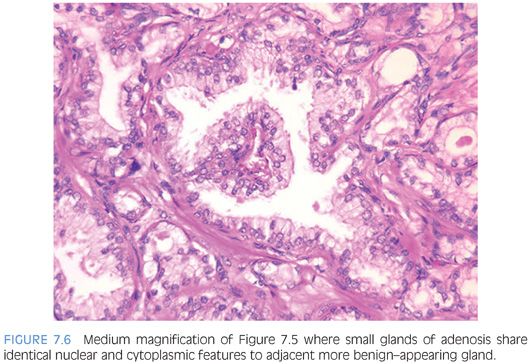
Probably the most important differentiating feature of adenosis seen on hematoxylin and eosin (H&E) stain is that within a nodule of adenosis there are elongated glands with papillary infolding and branching lumina typical of more benign glands, yet in their nuclear and cytoplasmic features, they look similar to the adjacent small glands suspicious for carcinoma (Figs. 7.6 and 7.7). Another common feature seen is the budding off of glands of adenosis from obviously benign glands. Glands of adenocarcinoma, even in the unusual case when the tumor is fairly lobular, shows a pure population of small crowded glands without benign architectural features that do not merge in with adjacent larger benign glands.
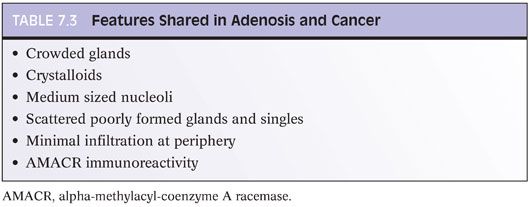
At higher power, adenosis is typically composed of small glands with pale to clear cytoplasm, as opposed to some carcinomas, which have more amphophilic cytoplasm (Figs. 7.7 to 7.9). In order for this feature to be diagnostically useful, the cytoplasm of benign prostate glands should appear pale or clear on routinely stained slides. A diagnosis of carcinoma should not be rendered based on what appears to be either a few individual cells or poorly formed glands within a nodule that is otherwise typical of adenosis. Occasional single cells or poorly formed glands are not uncommon in a nodule of adenosis and probably represent tangential sections of small glands (Table 7.3).
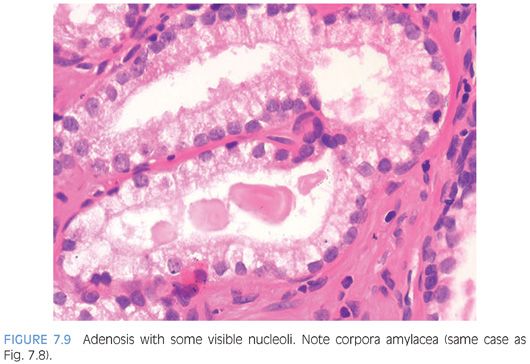
Usually, adenosis has been described as having totally bland-appearing nuclei without nucleoli. This is generally valid; most (60%) lesions contain no or at most rare prominent nucleoli. In the other 40%, fairly prominent (>1.6 microns) nucleoli are present, which should not lead to the diagnosis of carcinoma (Fig. 7.9).10
In another study, 18% contained nucleoli larger than 1 micron.1 Only huge nucleoli (>3 microns) are incompatible with a diagnosis of adenosis. In contrast, the majority (70%) of foci of low-grade adenocarcinoma have occasional or frequent large nucleoli. The remaining low-grade carcinomas have either no prominent or at most rare prominent nucleoli. These findings emphasize that, although nucleoli are generally helpful in differentiating adenosis from adenocarcinoma, there is overlap between the two entities.
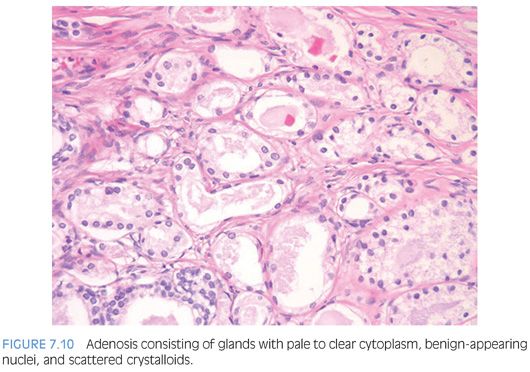
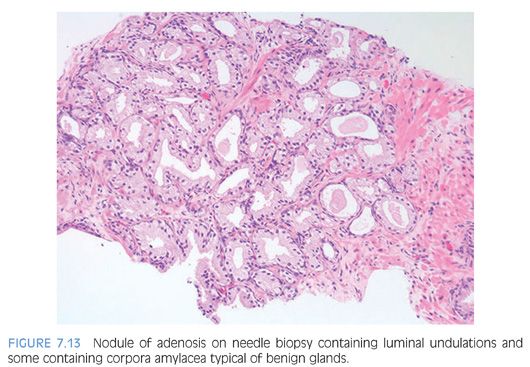
The luminal contents also may be useful in this differential diagnosis. Corpora amylacea are commonly seen in adenosis and are rare in carcinoma. Only 2% of cases of adenosis contain blue intraluminal secretions visible on H&E-stained sections, a feature common in low-grade carcinomas. It is not helpful to perform special stains for mucin. Despite earlier studies’ claims that acid mucin was diagnostic of carcinoma, a later work found that 54% of foci of adenosis contained acid mucin secretions.11 Crystalloids are intraluminal structures that have been touted as distinguishing adenosis from carcinoma. However, 18% to 39% of foci of adenosis contain crystalloids, sometimes in great number (Fig. 7.10). Crystalloids should not be used to differentiate adenosis and carcinoma (Table 7.3).
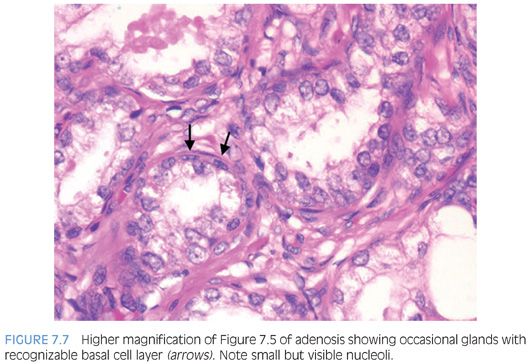
The presence of basal cells is the one feature seen in adenosis that is typically not seen in carcinoma. Although basal cells may be difficult to identify within many of the glands, a flattened basal cell layer can be seen in at least some of the glands. As long as the glands with a basal cell layer are otherwise identical to the glands where a basal cell layer cannot be identified, then the entire lesion is benign. It is important to distinguish basal cells from adjacent fibroblasts. Although fibroblasts have elongated, pointed, hyperchromatic nuclei, basal cell nuclei that are recognizable in routine sections have a more cigar-shaped ovoid contour with chromatin similar to that of the overlying secretory cells (Fig. 7.7). Basal cells may sometimes be apparent as a cluster of cells with scant cytoplasm polarized at the edge of a gland. In foci of glandular crowding where all of the features are typical of adenosis and there is no cytologic atypia, adenosis can be diagnosed without immunohistochemical stains even if basal cells are not visible on routine sections.
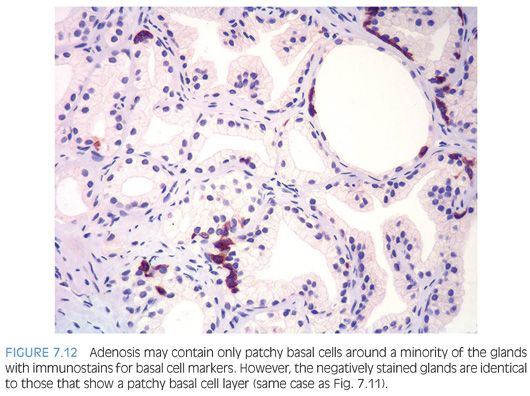
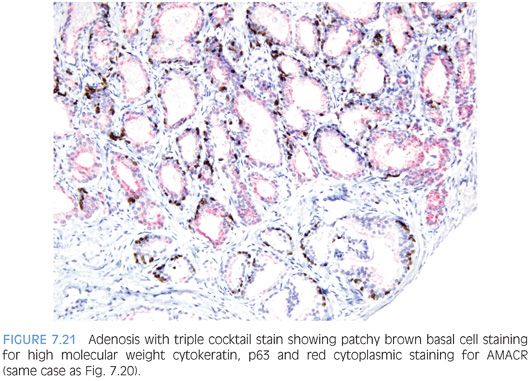
In cases where the architectural pattern favors adenosis yet there are visible nucleoli, the diagnosis can be clarified using immunohistochemistry for basal cells. The use of a basal cell specific antibodies to high molecular weight keratin or p63 is helpful since some glands will show a thin rim of keratin immunoreactivity beneath the cuboidal or columnar secretory cells.2,3,12 As few as 10% of the glands in a nodule of adenosis may be labeled with antibodies to basal cell markers, although usually more than half of the glands will show some staining. The stain is also patchy within a given gland, with sometimes only one to two basal cells identified (Figs. 7.11 and 7.12). If some glands suspicious for adenosis lack high molecular weight cytokeratin or p63 immunoreactivity, yet are otherwise indistinguishable from adjacent glands that demonstrate basal cell immunoreactivity, the absence of a basal cell layer in some glands should not be used to diagnose the lesion as carcinoma. Some of the variability in basal cell immunoreactivity within adenosis and other lesions may be caused by tissue fixation because more uniform immunoreactivity has been observed in frozen tissue. In addition to the patchy staining in adenosis, another immunohistochemical pitfall in the interpretation of these lesions is that they express racemase, a marker preferentially expressed in prostatic adenocarcinoma. In one study, 10% of cases demonstrated focal racemase positivity with 7.5% showing diffuse immunoreactivity.13
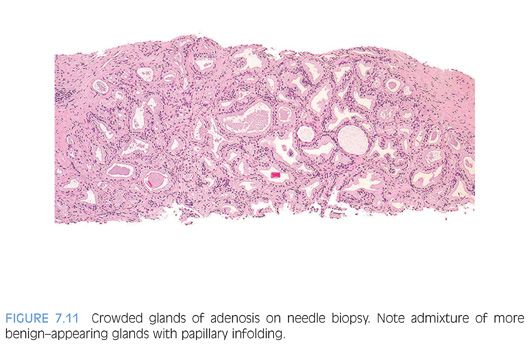
Adenosis often appears to be multifocal. In a few cases on TURP, foci are so numerous that, if misdiagnosed as carcinoma, they would be classified as stage T1b, leading to unwarranted radical therapy. The distinction between adenosis and low-grade adenocarcinoma in even a single focus may be critical, because diagnosis of even a single focus of carcinoma on TURP in a relatively young man may lead to aggressive surgery.
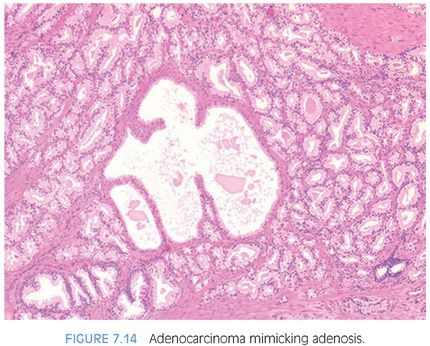
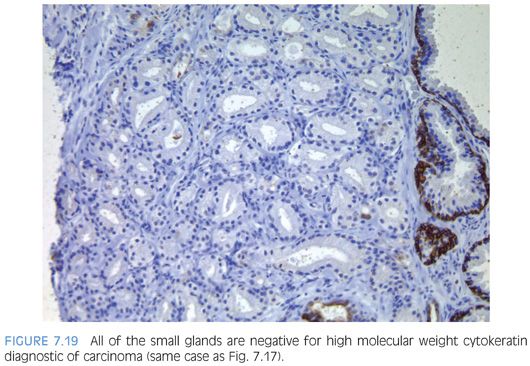
The diagnosis of adenosis on needle biopsy is more difficult, since it is more difficult to appreciate the architectural pattern on needle biopsy. Adenosis on needle biopsy appears as a relatively well-localized nodule of closely packed glands with pale to clear cytoplasm (Figs. 7.11 to 7.13). In only 7% of foci is the entire lobular lesion visualized on needle biopsy.3 In 45% of foci, one edge of the nodule can be appreciated and is circumscribed, yet the other side is not visible because the lesion is bisected by one edge of the needle biopsy. The remaining 48% of foci are transected in the middle of the nodule of adenosis such that the lesion extends to both edges of the needle biopsy. Although in these cases assessment of circumscription is difficult, in all but a few cases foci of adenosis occupy a small portion of the core length, uncommonly measuring more than 3 mm of the core length. Other than not having an entire nodule available for evaluation, the histologic features of adenosis on needle biopsy are the same as on TURP. On needle biopsy, due to the limited number of glands in question, basal cell specific antibodies must be interpreted with caution. Because basal cell staining may be patchy in adenosis, negative staining in a small focus of glands is not necessarily indicative of malignancy. However, if some of the glands within a crowded glandular focus on needle biopsy demonstrate a basal cell layer, then adenosis can be diagnosed. Because of the difficulty in diagnosing adenosis on needle biopsy, it is useful to verify the diagnosis with high molecular weight cytokeratin or p63 antibodies. In the evaluation of a nodule of adenosis, it is difficult to determine where the smaller crowded glands, where one is considering the diagnosis of cancer, end and the more obviously benign glands begin, because the small glands of adenosis merge in with the surrounding more recognizable benign glands. In contrast, with cancer, one should be able to identify each gland in question as malignant based on cytologic and/or architectural differences compared to adjacent benign glands. Whereas the immunohistochemical staining pattern in adenosis shows a few glands with patchy basal cell staining, cancer glands are negative and adjacent benign glands show circumferential complete basal cell immunoreactivity (Figs. 7.14 to 7.21).
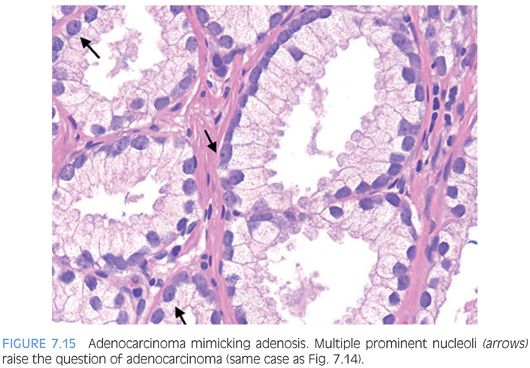
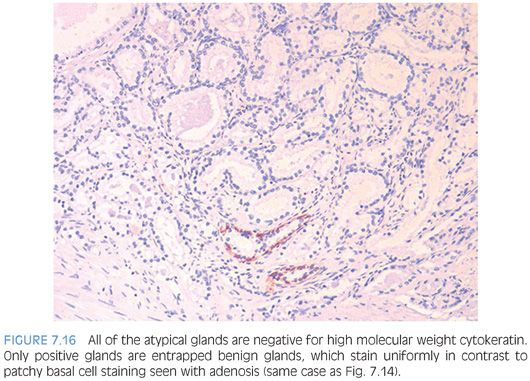
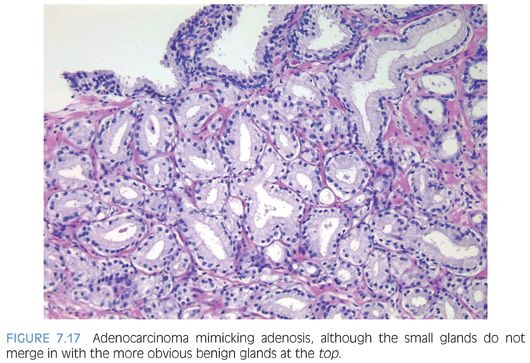
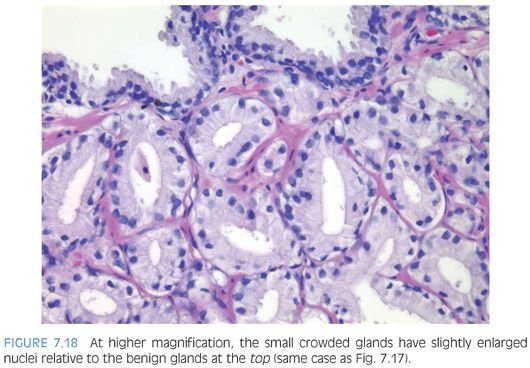
Although adenosis mimics carcinoma, there is no conclusive evidence suggesting that patients with adenosis have an increased risk of harboring or developing adenocarcinoma of the prostate. In one series of adenosis, 14% of the transurethral resection (TUR) specimens examined also contained incidental foci of adenocarcinoma of the prostate.2 This is similar to the reported frequency of incidental adenocarcinomas found in TURs performed for clinically benign disease. Prior reports of transitions between adenosis and carcinoma were not verified with the use of basal cell specific antibodies and may have been adenosis with foci of individual cells, minimal infiltration, or visible nucleoli. Another argument that has been raised to suggest that adenosis is a precursor to prostate cancer is that the two entities share certain morphologic features. Several studies have shown that adenosis may contain acid mucin, crystalloids, nucleoli, racemase, and have a patchy basal cell layer. Rather than proving a relation between adenosis and carcinoma, these findings demonstrate that any one of these features, by itself, is not specific for carcinoma. For example, acid mucin may be seen in atrophy a patchy basal cell layer in clear cell cribriform hyperplasia, racemase in partial atrophy, and nucleoli in basal cell hyperplasia.9,11,14,15 None of these lesions is considered a precursor to prostate cancer. The interpretation of these features must be made in the context of the totality of a lesion’s architectural and cytologic features. Those studies suggesting a higher risk of carcinoma in men with adenosis have defined it differently, including many examples of what most authorities would call carcinoma.16 Adenosis is closer to benign prostatic hyperplasia than carcinoma in terms of its proliferation rate.17,18 There have been a limited number of studies looking at the genetic findings in adenosis. Qian et al.,19 using fluorescence in situ hybridization (FISH) analysis, demonstrated chromosomal anomalies in only 9% of cases of adenosis as compared to 55% of carcinomas. There was also no relationship between the chromosomal anomalies seen in adenosis and matched foci of carcinoma. In another study by the same group, Cheng et al.20 noted allelic imbalances in 7 of 15 (47%) cases of adenosis. A subsequent study by Doll et al.,21 however, found allelic imbalances in only 12% of cases of adenosis. One potential difference between the two studies was that the cases with foci of adenosis in the study by Doll et al.21 lacked associated carcinomas. Also, Doll et al.21 used the more stringent allelic imbalance criteria of a 50% reduction of allelic intensity in adenosis samples as compared to the patient-matched normal samples, whereas Cheng et al.20 used a 30% reduction criterion. Bettendorf et al.,22 using comparative genomic hybridization, found that adenosis uncommonly had allelic imbalances and concluded that adenosis is not closely linked to prostatic carcinoma. These cumulative results suggest that genetic alterations in adenosis may be infrequent.
In a more recent study by Cheng et al.,23 TMPRSS2-ERG gene fusion (see Chapter 19), a common chromosomal rearrangement that occurs early in the development of invasive adenocarcinoma of the prostate and is present in 50% of adenocarcinomas and in 20% of high-grade prostate intraepithelial lesions, were assessed in adenosis by FISH and immunohistochemistry techniques. None of the 55 prostatic adenosis specimens that were investigated showed evidence of TMPRSS2-ERG alteration by either technique.23 Similar results were also found by our group.24 Formalin-fixed, paraffin-embedded tissue sections of adenosis from cases of prostate biopsies (n = 30), TURPs (n = 12), and radical prostatectomies (n = 3) were analyzed using immunohistochemistry for ERG. None of the foci of adenosis were positive for ERG protein expression. In comparison, in 40 cases of Gleason score 6 adenocarcinoma on a tissue microarray, 22 (55%) were positive for ERG protein. The findings in both studies support the notion that adenosis is not a precursor lesion of adenocarcinoma. Moreover, it suggests that immunohistochemistry for ERG expression could be a useful tool to differentiate adenosis from adenocarcinoma.24
The most critical issue in terms of patient management is whether patients with adenosis on histologic examination are at increased risk of subsequently being diagnosed with adenocarcinoma. In the only study to address this issue, Renedo et al.25 studied 24 men with foci of adenosis compared to 61 men with benign prostatic hyperplasia. Men with adenosis were followed on average 6.5 years. There was no difference in the subsequent development of adenocarcinoma between the two groups. When diagnosing adenosis, we include the following statement, “Adenosis, although mimicking cancer, has not been shown to be associated with an increased risk of prostate cancer.”
DIFFUSE ADENOSIS OF THE PERIPHERAL ZONE
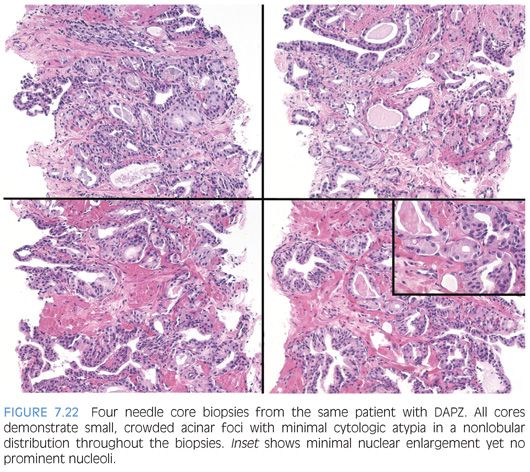
We have observed a group of typically younger patients with multiple foci of small, nonlobular, crowded, but relatively bland acini on needle biopsy as well as in prostatectomy specimens.26 The architectural pattern can mimic low-grade adenocarcinoma especially in the subset of cases that may display rare acini with cytologic atypia. It is unclear whether this architectural pattern, which we have termed diffuse adenosis of the peripheral zone (DAPZ), is simply a crowded glandular variant of normal prostate morphology or whether it represents a precursor or a risk factor for the development of prostatic carcinoma. Men with DAPZ tend to be younger (mean age: 49 years; range: 34 to 73 years) than the average age of men with prostate cancer. We evaluated 60 such cases on needle biopsy. Over half of the men on rebiopsy cases (57%) were subsequently diagnosed with carcinoma. Although the majority of tissue sampled in a typical DAPZ case had no cytologic atypia, in two-thirds of cases there were admixed rare foci of atypical glands with prominent nucleoli comprising less than 1% of submitted tissue. Patients with a subsequent diagnosis of carcinoma were more likely to have had DAPZ with focal atypia. DAPZ should be considered a risk factor for prostate cancer and that patients with such finding should be followed closely and rebiopsied (Fig. 7.22).26
ATROPHY
Typically considered to be a process affecting the elderly, atrophy has been demonstrated in at least 70% of 19- to 29-year-old men.27 Atrophy may result in prostatic induration or give rise to a hypoechoic lesion on transrectal ultrasound and may be biopsied as a lesion suspicious for cancer.
There are distinct histologic variants of atrophy, which can be classified as simple atrophy, postatrophic hyperplasia (PAH), and partial atrophy.28 At low magnification, glands of simple atrophy appear basophilic, which reflects relative lack of cytoplasm both apically and laterally compared to normal epithelium. Simple atrophy glands are of relatively normal caliber and are generally spaced apart in a configuration similar to that of normal epithelium. In simple atrophy with cyst formation, the acini are rounded and appear cyst-like. Many of the acini in this pattern are arranged in a back-to-back configuration with little intervening stroma. Simple atrophy does not pose diagnostic difficulties.
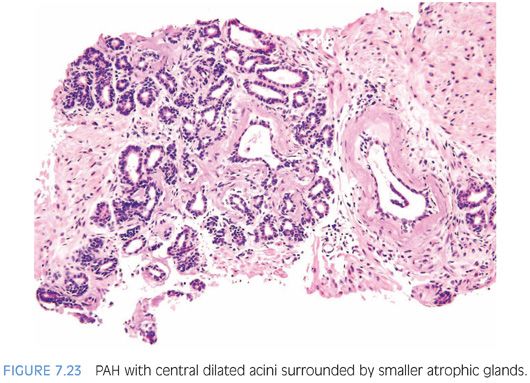
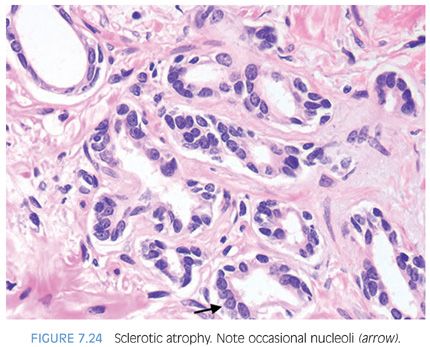
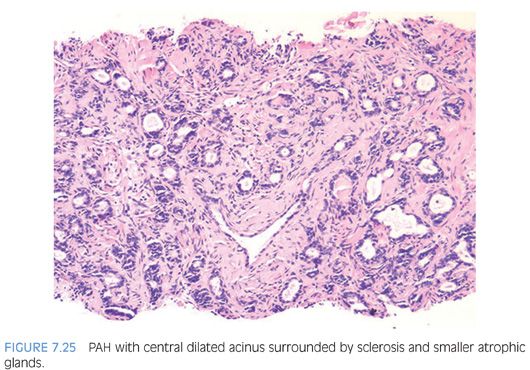
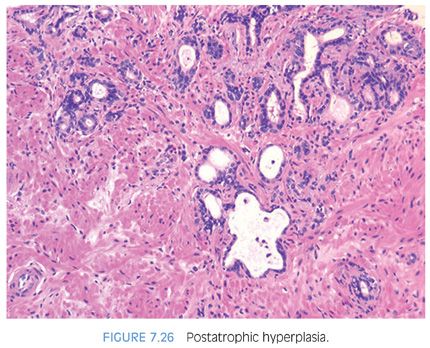
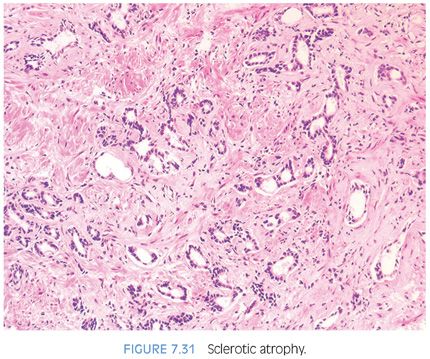
PAH also often appears basophilic at low power. It consists of acini that are small and mostly round that are arranged in a lobular distribution.29 Often, these acini appear to be surrounding a somewhat dilated “feeder” duct (Figs. 7.23 to 7.25). Many of these lesions frequently resemble normal-appearing resting breast lobules and are referred to by some authors as lobular atrophy. The lesions appear hyperplastic because the close packing of multiple small acini suggests that there is an increase in their number compared to normal tissue. PAH glands have a much higher proliferation rate than nonatrophic benign glands, and in some cases, mitotic figures can be identified (Figs. 7.26 and 7.27).30 Although the glands may appear infiltrative, they appear invasive as a patch not as individual glands infiltrating in between larger benign glands. The basophilic appearance of glands of atrophy is due to their scant cytoplasm and crowded nuclei such that at low magnification one is merely seeing a nuclear outline of the gland (Figs. 7.28 and 7.29, eFigs. 7.116 to 7.149). Longitudinal tangential sections of atrophic glands results in cords of cells that can further mimic cancer (Fig. 7.30, eFigs. 7.150 to 7.152). In some cases, there may be associated fibrosis, which gives the atrophic glands a more infiltrative appearance that has been termed in the past as sclerotic atrophy (Fig. 7.31). Whether one uses the term postatrophic hyperplasia or merely benign prostate tissue with atrophy in one’s diagnostic reports is a matter of personal preference.
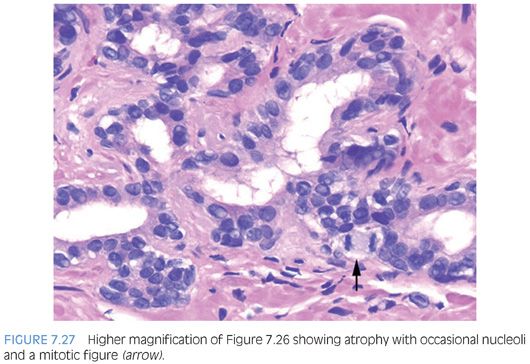
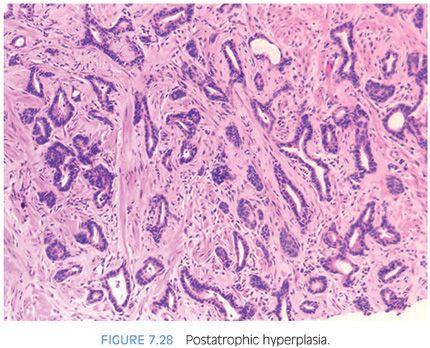
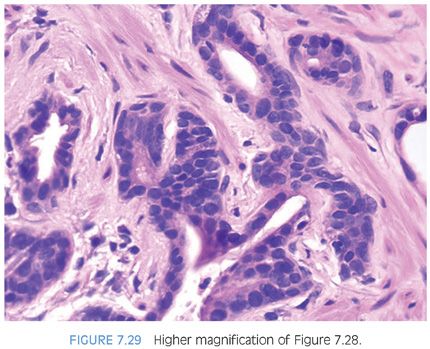
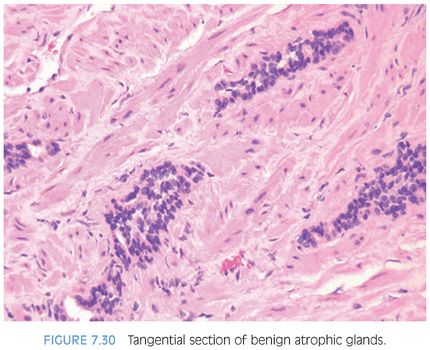
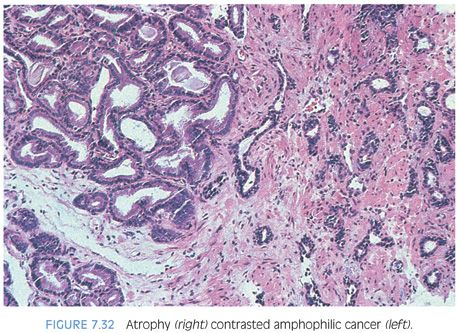
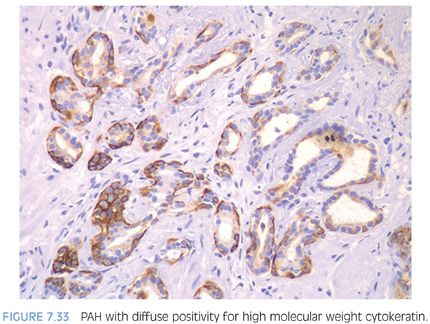
Compared to atrophy, gland-forming adenocarcinomas of the prostate typically have a greater amount of cytoplasm so that at low magnification, the neoplastic glands are not as basophilic. Atrophy’s very basophilic appearance is distinctive even when compared to adenocarcinoma with very amphophilic cytoplasm (Fig. 7.32). Atrophy may show enlarged nuclei and prominent nucleoli, although not the huge eosinophilic nucleoli seen in some prostate cancers. Although prominent nucleoli are more common in atrophic glands associated with inflammation, we have also seen prominent nucleoli in atrophy without inflammation. Furthermore, the inflammation associated with atrophy may be trivial and chronic in nature but still give rise to significant nuclear atypia. In deciding whether an atypical focus represents carcinoma, the presence of atrophic cytoplasm should, in general, make one cautious in diagnosing carcinoma. When there are concerns as to whether a focus represents PAH or adenocarcinoma, immunohistochemistry with antibodies to high molecular weight cytokeratin or p63 can be performed to resolve the issue, as PAH uniformly labels with basal cell markers (Fig. 7.33). As opposed to partial atrophy (see the following text), PAH uncommonly expresses racemase.31,32
Rarely, carcinoma with an atrophic appearance may be present on needle biopsy. The diagnosis of carcinoma in these cases is made on (a) a truly infiltrative process with individual small atrophic glands situated between larger benign glands; (b) the concomitant presence of ordinary, less atrophic carcinoma; and (c) greater cytologic atypia than is seen in benign atrophy (see Chapter 6).31
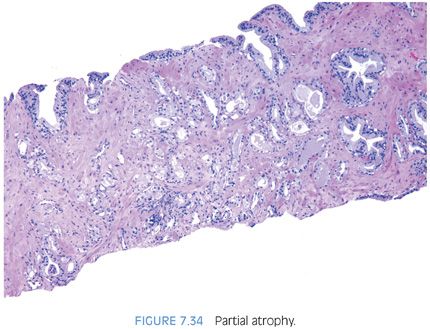
Another variant of atrophy, the most common mimicker of prostate cancer that causes confusion with carcinoma, is “partial atrophy”9,33 (eFigs. 7.153 to 7.201). Partial atrophy may still retain the lobular pattern of PAH, or as seen in Figures 7.34 and 7.35, have more of a disorganized diffuse appearance. Partial atrophy lacks the basophilic appearance of fully developed atrophy (simple atrophy, PAH) as the nuclei are more spaced apart (Figs. 7.36 to 7.38). The presence of crowded glands with pale cytoplasm may lead to an overdiagnosis of low-grade adenocarcinoma. At higher power, however, the glands have benign features characterized by undulating luminal surfaces with papillary infolding. Most carcinomas have more straight, even luminal borders. In addition, the glands are partially atrophic with nuclei in areas reaching the full height of the cytoplasm. The nuclear features in partial atrophy tend to be relatively benign without prominent nucleoli, although nuclei may appear slightly enlarged with small nucleoli. One should hesitate diagnosing cancer when the nuclei occupy almost the full cell height and the cytoplasm has the same appearance as surrounding more obvious benign glands. As with adenosis, partial atrophy typically has a patchy basal cell layer and express racemase (Fig. 7.39).9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


