CHAPTER 11
Long-Acting Injectable Antipsychotics
MICHAEL BIGLOW, BS, PharmD, BCPS, BCPP
MEGAN FLINCHUM, PharmD, BCPS
DRUG CLASS OVERVIEW
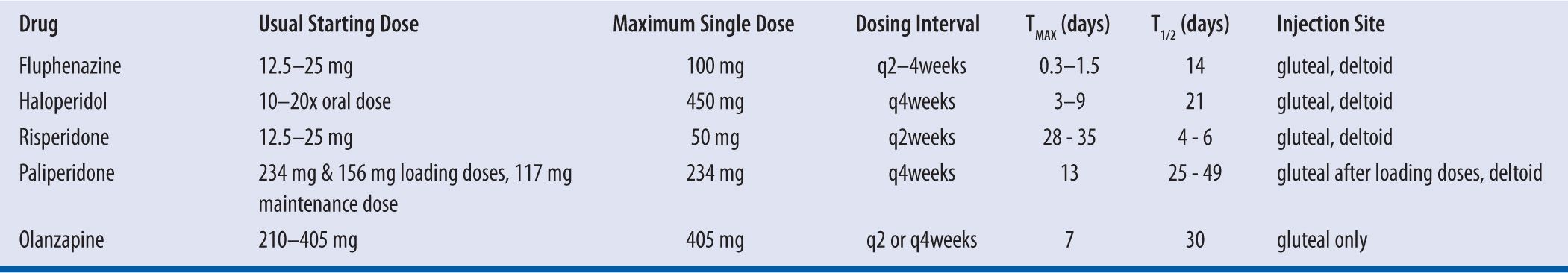
Antipsychotics are the mainstay of drug treatment for the management of schizophrenia and other psychotic disorders. The mechanism of action of the medication depends on the type of antipsychotic with the older “typical” antipsychotics focusing on the antagonism of postsynaptic dopamine type-2 (D2) receptors. The newer “atypical” drugs antagonizing both D2 and serotonin type-2A receptors elicit a comparable antipsychotic effect but with the potential for lessening iatrogenic movement disorders and improving negative symptoms (e.g., anhedonia, flattened affect, cognitive impairment).1 The option of using depot formulations of these medications allows for a number of benefits including consistent drug delivery, assured patient compliance, predicable bioavailability, and avoidance of intentional or accidental overdose.2 Available depot formulations exist for the typical antipsychotics haloperidol and fluphenazine and the atypical antipsychotics risperidone, paliperidone, and olanzapine. Dosing parameters vary due to different drug release mechanisms and intended time to response. (see Table 11-1) Their role in therapy has been established and they are recommended for patients who would prefer this method of treatment with the simplification of medication administration, who have a history of relapse due to noncompliance, and when avoiding noncompliance is a clinical priority.2-4 Although patients on depot antipsychotics receive treatment on a more consistent and monitored basis, data are still limited on whether depot injections reduce relapse rates or long-term adverse drug events compared to oral antipsychotics.5,6
| TABLE 11-1 | Dosing Parameters of Long-Acting Injectable Antipsychotics |
TYPICAL ANTIPSYCHOTICS
HALOPERIDOL AND FLUPHENAZINE
Haloperidol and fluphenazine are the two typical, or first-generation, antipsychotics available in a long-acting injectable form. Both are synthesized via esterification to a long chain fatty acid, decanoate. Previously, ethanate had been utilized as a lipid chain for fluphenazine but this formulation is no longer available in the United States. The esters are then dissolved in purified sesame oil for final preparation in the standard concentrations of 50 mg/mL and 100 mg/mL for haloperidol decanoate and 25 mg/mL for fluphenazine decanoate.7,8,9 After intramuscular injection, the availability of the drug is presumed to be dependant on diffusion from the sesame oil because it has been observed that the hydrolysis of the ester is rapid and enzymatically mediated. The free drug is then allowed to pass through the blood brain barrier and elicit its antipsychotic effect (see Figure 11-1). Since the elimination rate constant remains the same after conversion to active drug, the absorption rate constant is the rate-limiting kinetic step, which has been described as a “flip-flop” kinetics model.10 Therefore, the time to steady-state concentration is dependent on the absorption and could take as long as 3 months to achieve.
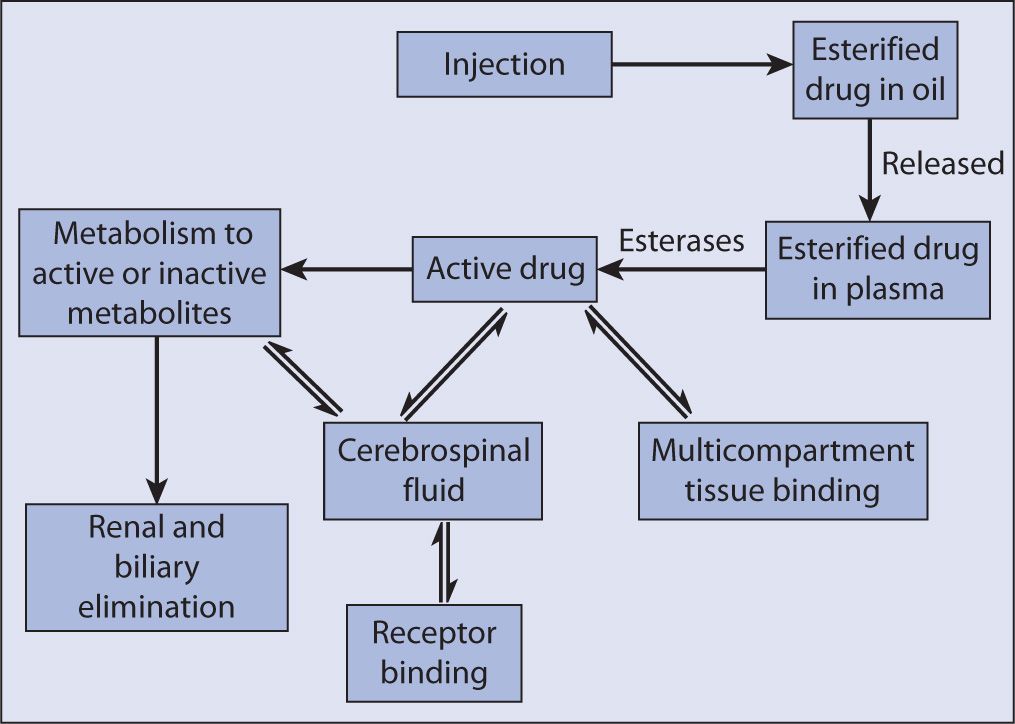
FIGURE 11-1. Disposition of depot antipsychotics. Source: Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315–333.
Therapeutic Concentrations
Therapeutic plasma concentrations for both haloperidol and fluphenazine have been proposed, ranging from 5–14 ng/mL for haloperidol and <0.15–0.5 ng/mL for fluphenazine, but routine monitoring is not an established practice due to wide patient variability in response.9,10,11 A linear correlation between haloperidol decanoate dose and steady-state plasma concentration has been established with a linear regression equation9:
Plasma concentration (ng/mL) = 0.0291 × haloperidol decanoate
dose (mg/month)
Effective doses generally require 60–80 percent of postsynaptic D2 receptors to be antagonized, with lower percentages being less effective, except in the case of clozapine, and higher percentages being more associated with extrapyramidal symptoms (EPS).12,13
Bioequivalence to Oral Therapy
Due to the nature of the route of drug administration, depot administration bypasses oral absorption variability and first-pass or other predistribution metabolism. The conversion of stabilized patients to haloperidol decanoate can utilize a loading dose of 20 times the daily oral dose for the first injection and 10–15 times the oral dose for subsequent doses, or the prescriber can opt to give 10–15 times the oral dose but it is recommended to continue the oral dose of haloperidol for at least seven days. Fluphenazine decanoate is recommended to be dosed at 12.5–25 mg initially although a conversion ratio has been calculated at 1.6 times the daily oral dose being equivalent to the intramuscular fluphenazine decanoate requirement in mg/week but this conversion is rarely utilized.10
Clearance
Both medications undergo extensive hepatic metabolism with single-dose kinetic studies showing that after an initial peak within 24–48 hours, fluphenazine follows first-order elimination kinetics with an apparent half-life of 6.8–9.6 days, which increases to 14 days after multiple injections. Haloperidol decanoate has a peak after 7 days (range 3–9 days) and is noted to have a half-life of approximately 3 weeks.9,10
ATYPICAL ANTIPSYCHOTICS
RISPERIDONE
The first atypical or second-generation antipsychotic to be available in a long-acting injectable (LAI) formulation is risperidone. It differs significantly from the first depot injections in that it is an aqueous formulation containing drug microencapsulated in a polylactide-co-glycolide (PLGA) polymer.14 The polymer slowly hydrolyzes, releasing the drug in a slow but steady absorption pattern with clinically significant plasma levels of risperidone and its active metabolite, paliperidone (9-hydroxyrisperidone), developing in 3 weeks.
Therapeutic Concentration
After initial drug release, plasma concentration of risperidone continues to rise, reaching CMAX in 4–5 weeks and lasting up to 7 weeks. This delay in drug absorption after the first injections necessitates the need for oral overlap for the first 3 weeks, although some practitioners would recommend covering for at least 6 weeks or after four injections to ensure the subject is at steady state. Kinetic studies (see Table 11-2) have shown that oral doses of 2 mg, 4 mg, and 6 mg daily are equivalent to 25 mg, 50 mg, and 75 mg of long-acting risperidone every 2 weeks, respectively, but current dosing conversion recommendations are 25 mg, 37.5 mg, and 50 mg every 2 weeks based on dose-repsonse studies.15
| TABLE 11-2 | Steady-State Pharmacokinetics and Bioavailability of Long-Acting Risperidone Injection and Oral Risperidonea |
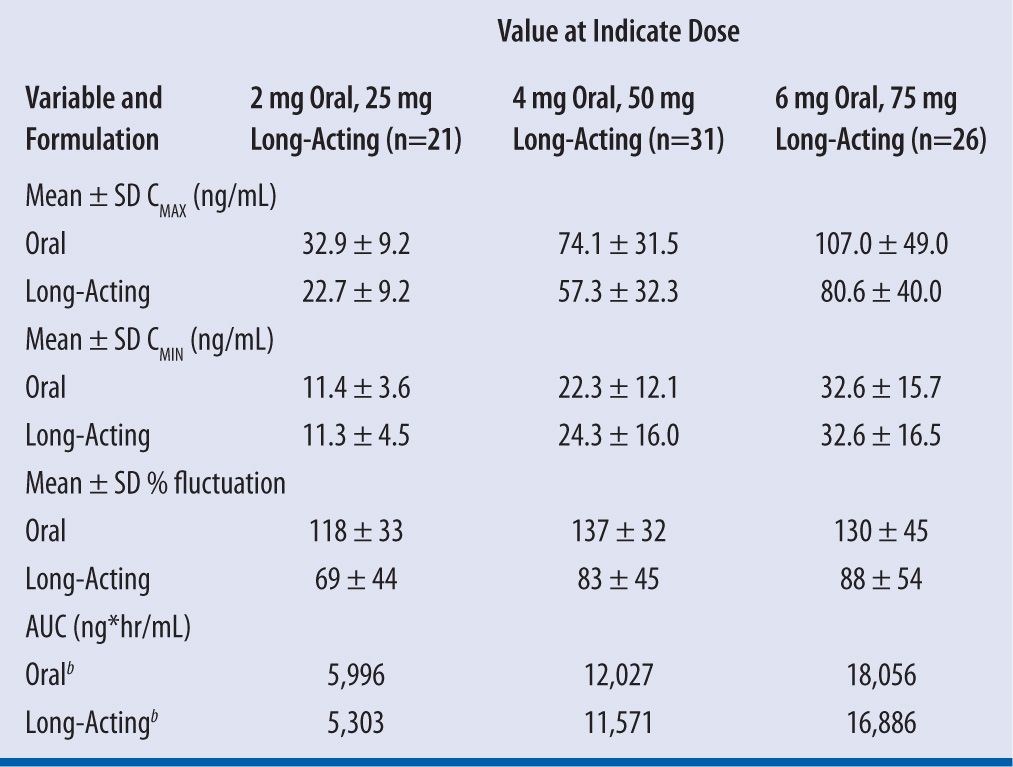
aCMAX = maximum plasma drug concentration, CMIN = minimum plasma drug concentration, AUC = area under the concentration-versus-time curve.
bLeast squares means (log transformed).
Source: Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70(1):91–100.
Bioequivalence to Oral Therapy
Bioequivalent conversion from oral to risperidone LAI as noted already has shown a slight decrease in AUC with an IM to oral ratio ranging from 88–94 percent as the dose increases from 25 mg to 75 mg.15
Clearance
As the drug is released from the polymer matrix the pharmacokinetics of oral and long-acting risperidone are similar. The metabolism of risperidone to 9-hydroxyrisperidone is mediated via cytochrome P-450 (CYP) isoenzyme 2D6. Due to the difference in drug-release mechanism, the half-life of risperidone LAI is increased from 3 hours to 4–6 days.19
PALIPERIDONE
Paliperidone is another atypical antipsychotic developed into a long-acting injection formulation.20 However, the formulation of paliperidone palmitate differs from the long-acting injection formulation of risperidone. Paliperidone palmitate is an extended-release aqueous-based nanosuspension, whereas risperidone has the unique microsphere formulation.21,14
Paliperidone palmitate has a low solubility and allows for an extended-release injectable product.21 The isotonic aqueous buffer in which the drug is suspended penetrates muscle tissue and leaves a collection paliperidone palmitate particles locally at the injection site.21,22 The drug particles dissolve slowly and are hydrolyzed into paliperidone and palmitic acid exhibiting biphasic absorption into systemic circulation.21 The extended-release profile is a direct function of the paliperidone palmitate particle size, which is controlled by the wet grinding manufacturing process to increase surface area.21
Therapeutic Concentration
Paliperidone palmitate is initiated with a 234 mg injection on Day 1, and a 156 mg injection on Day 8 with monthly injections thereafter.22 A paliperidone plasma concentration of 7.5 ng/mL, the threshold for antipsychotic efficacy, is generally reached within one week after the first injection.23 However, plasma concentrations do not approach the CMAX of 19 ng/mL until Day 13 after paliperidone palmitate initiation.21 Due to its biphasic release pattern, supplemental oral antipsychotic doses are not recommended once the first dose of paliperidone palmitate is administered.22,23 Previous kinetic studies have shown that monthly intramuscular paliperidone palmitate plus daily oral paliperdone doses of 6 mg or 12 mg daily results in a CMAX range of 55–80 ng/mL.22 Daily doses of 3 mg, 6 mg, and 12 mg are equivalent to monthly intramuscular paliperidone palmitate doses of 39 mg/78 mg, 117 mg, and 234 mg, respectively.20
Bioequivalence to Oral Therapy
Various kinetic trials have shown that median peak concentrations are 28 percent higher after the first injection into the deltoid muscle, compared to the gluteal muscle, which is most likely due to general anatomy of each site with more muscle and less adipose tissue within the deltoid.20,22 For this reason, it is recommended to initiate paliperidone palmitate in the deltoid muscle to achieve therapeutic concentrations more rapidly.22 However, the overall AUC resulting from both injection sites are within comparable ranges.22 So when administering maintenance injections, it is acceptable to rotate injection sites.22
Bioequivalent doses of daily oral paliperidone to monthly intramuscular paliperidone palmitate are as mentioned previously. It is important to note that 156 mg of paliperidone palmitate is equivalent to 100 mg of active paliperidone. See Table 11-3 for equivalency conversions from long-acting intramuscular risperidone to paliperidone palmitate.23
| TABLE 11-3 |



