CHAPTER 10
Lithium
HENRY COHEN, MS, PharmD, FCCM, BCPP, CGP
Lithium was discovered in 1818, and its psychiatric benefits were discovered in the 1940s. Until 1950, the popular beverage 7-Up contained lithium citrate and was positioned for people with hangovers. The number “7” in 7-Up is in reference to the atomic mass of lithium and the word “Up” is in reference to the uplifting effects of the lithium citrate.
Lithium is indicated for the management of bipolar disorders, the acute treatment of manic episodes or mixed episodes in patients with bipolar 1 or bipolar 2 disorder, and maintenance therapy in bipolar disorders to prevent or decrease the intensity of subsequent manic episodes.1-5 Lithium is also indicated for refractory unipolar depression (60–80% efficacy).3 Lithium has also been used for the management of bulimia, tardive dyskinesia, alcoholism, cluster headaches, postpartum psychosis, corticosteroid psychosis, posttraumatic stress disorder, aggression, as an augmentation agent for patients with depression, disorders of impulse control, schizoaffective and schizophrenic disorders, neutropenia or anemia, and hyperthyroidism.3-6 Lithium has been used for the syndrome of inappropriate antidiuretic hormone, however, due to the perils of using lithium in patients with water imbalances and the availability of demeclocycline and the newer vaptans such as conivaptan, lithium should only be used as a refractory agent.
Lithium has several mechanisms of action that influence its clinical effects in psychiatry. Lithium reduces cation transport such as calcium, magnesium, sodium, and potassium into cell membranes in the nerves and muscles.2,3 These univalent and divalent cations are involved in the synthesis, storage, release, and reuptake of catecholamines. Lithium also reduces the reuptake of catecholamines and attenuates supersensitive receptors, resensitizing the receptor and reestablishing the effects of norepinephrine, epinephrine, serotonin, and dopamine.3 Both norepinephrine and dopamine may be involved in the pathogenesis of mania, and serotonin may be involved with depression. The effects of lithium may be noted within 7–14 days, and 14–21 days for a full effect.
THERAPEUTIC AND TOXIC PLASMA CONCENTRATIONS
Lithium has a narrow therapeutic index but a well-defined plasma concentration range. The usual lithium target serum level for acute manic or mixed episodes in patients with bipolar 1 or bipolar 2 disorder is 0. 8–1.2 mEq/L; rarely levels of 1.2–1.5 mEq/L are needed.6 Once the patient’s manic episode is stabilized, maintenance lithium serum levels are 0.6–1.0 mEq/L and rarely 1.0–1.2 mEq/L. In order to minimize lithium-adverse effects, the target ranges of lithium for the elderly are usually 0.2 mEq/L or less.7 The available target serum levels for lithium assume a multiple daily dose model; no target level has been established for once-daily dosing.
ADVERSE EFFECTS
Two of the most common adverse effects associated with lithium are gastrointestinal and central nervous system (CNS) related, and generally resolve with continued treatment.2 Gastrointestinal side effects may occur in up to 30 percent of patients and include nausea, vomiting, diarrhea, and bloating and are more problematic with the extended release lithium dosage forms.8 Central nervous system adverse effects occur in 40–50 percent of patients, and include confusion, lethargy, fatigue, headache, mild memory impairment, muscle weakness, and tremor.8 The hand tremor occurs in up to 50 percent of patients and manifests as a fine, rapid intention tremor.9,10 The CNS adverse effects of lithium may be associated with high peak levels, and may be minimized by administering the immediate-release lithium products with food or by using the extended-release lithium products.11
Lithium may reversibly increase the WBC count by 10–30 percent and has been used to treat neutropenia secondary to a variety of causes, with well-controlled studies completed in patients with antineoplastic drug-induced neutropenia.3,12-13 Lithium-induced leukocytosis, with leukocyte counts of 10,000–15,000/mm3 have been observed.3 Lithium should not be used in patients with leukemia.
Lithium decreases the response to antidiuretic hormone (arginine vasopressin) and may cause nephrogenic diabetes insipidus (DI). The incidence of lithium-induced DI is 30–50 percent and occurs shortly after treatment is started and persists in 10–25 percent with chronic treatment.14 Polyuria followed by polydipsia and xerostomia occur with increased urine volumes to greater than 5 L/day. Polyuria has been successfully ameliorated with the potassium-sparing diuretics amiloride or triamterene.15
Lithium may cause hypothyroidism—the incidence is 1–4 percent. Lithium inhibits organification of iodine and inhibits conversion of tetraiodothyronine (T4) to triiodothyronine (T3). Elevated thyroid-stimulating hormone (TSH) occurs in 6–25 percent of patients.16,17 Patients may present with goiters, with or without hypothyroidism. All patients receiving lithium should be monitored for signs and symptoms of hypothyroidism such as fatigue, depression, brittle hair, coarse skin, cold intolerance, and hypotension. The TSH levels should be completed at baseline and monitored every 6–12 months. Other endocrine effects of lithium include mild asymptomatic hyperparathyroidism, and manifests with increased calcium and decreased phosphate serum levels.3
Lithium-induced dermatologic adverse effects occur in 1 percent of patients—acneform eruptions, folliculitis, and psoriasis exacerbation are most common.18,19 A Raynaud’s disease-like effect occurs rarely and after one day of use, presenting with painful discolored fingers and toes and coldness of extremities.19 Lithium may cause benign electrocardiogram changes, specifically T-wave inversion with an incidence of 30 percent.3 Lithium causes nonspecific renal morphologic changes such as glomerular fibrosis and interstitial fibrosis, nephron and tubular atrophy.20 Sclerosis of up to 10–20 percent of glomeruli have been observed in some patients. The relationship between these changes and renal function are unknown and generally have not been associated with decreased renal function.21 Nevertheless, it is prudent to monitor renal function with chronic lithium use.
Lithium is a teratogen and is classified as pregnancy category D.3 Lithium readily crosses the placenta and fetal lithium levels are equal to that of the mother. Lithium has caused an increased risk of Ebstein’s anomaly of the tricuspid valve to 1:1,000 from 1:20,000 in the normal population. Other lithium-induced cardiac anomalies such as ventricular conduction delay have been reported.22 Teratogenic effects seen with lithium include Down syndrome and club foot. Lithium should not be used in the first trimester because of the highest risk of teratogenic effects.23 If lithium is used during pregnancy, the lithium daily dose will have to be increased due to increased lithium clearance. Immediately, postpartum renal clearance of lithium decreases to prepregnancy levels, and the lithium daily dose will have to be reduced.
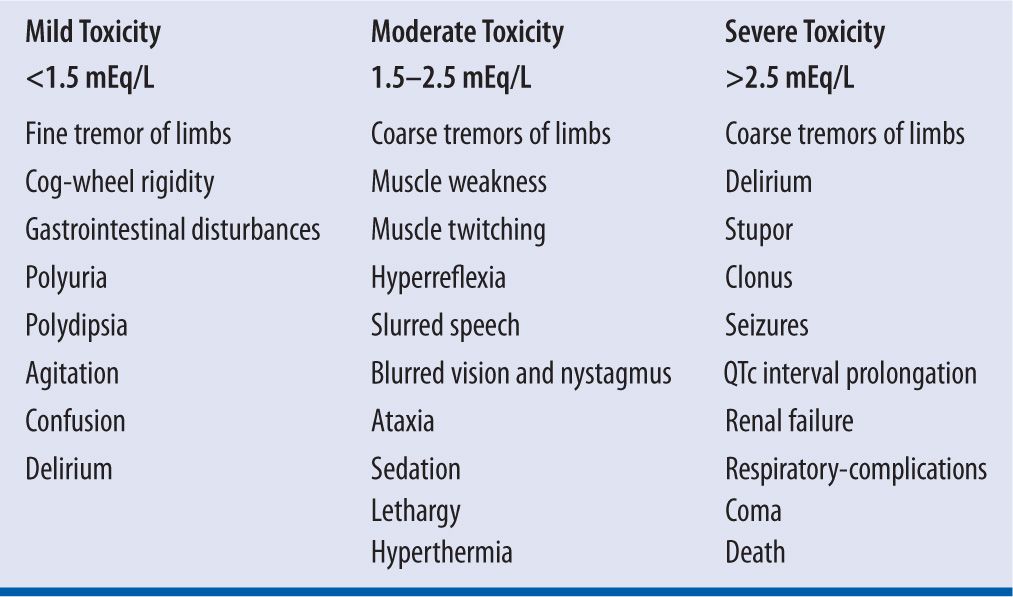
Lithium is a neurotoxin and toxicity can be life-threatening, presenting with coarse tremors, stupor, seizures, dysrhythmias, renal failure, coma, and death.24 Poor clinical outcomes with lithium toxicity can be predicted by the duration of lithium toxic exposure and can lead to permanent basal ganglia damage.25 The syndrome of irreversible lithium-effectuated neurotoxicity (SILENT) describes irreversible neurologic and neuropsychiatric sequelae from chronic lithium toxicity that persists for at least two months after lithium has been discontinued.26 See Table 10-1 for concentration-related toxicities of lithium. In order to avoid lithium toxicity patients should avoid scenarios that cause dehydration, such as excessive sun exposure, diarrhea, vomiting, fever, and diaphoresis. The loss of sodium and water will lead to reabsorption of lithium and lithium toxicity. Patients should be instructed to maintain a regular diet with special attention to sodium intake, drink 8–12 eight-ounce glasses of liquid daily, and maintain a daily fluid input at 2,500–3,000 mL.
| TABLE 10-1 | Concentration-Related Lithium Toxicity |
BIOAVAILABILITY
Lithium is not available intravenously, so the syrup liquid dosage form is used to determine bioavailability and is considered to be 100 percent bioavailable. Lithium is absorbed rapidly and achieves peak plasma concentrations with the liquid syrup dosage form within 30–60 minutes, with immediate-release tablets and capsules in 1–3 hours, and with sustained-release dosage forms in 3–12 hours.3 The gastrointestinal absorption from immediate-release dosage forms of lithium in tablets, capsules, or syrup is 95–100 percent, hence the bioavailability of lithium is 1 (F = 1).27 The absorption of the sustained release dosage forms is 60–90 percent, in clinical practice 80 percent (F = 0.8) may be used.3 Food decreases the peak plasma concentrations of lithium but does not decrease the bioavailability of lithium.
VOLUME OF DISTRIBUTION
Lithium is not protein bound and is widely distributed and approximately equal to that of body water. Lithium has a molecular weight of 74 daltons and is a monovalent cation. Lithium distribution follows a two-compartment open pharmacokinetic model. Lithium distributes rapidly to the central compartment, organs with a good blood supply (blood, heart, lungs, liver, and kidneys) and less rapidly to the peripheral compartments (fat, skin, muscle, bone, thyroid, and brain).28 The initial volume of distribution of lithium is 0.2–0.3 L/kg, and after distribution is complete the final volume of distribution is 0.7 L/kg. The range of the lithium volume of distribution is 0.6–1.2 L/kg. The lithium volume of distribution in the elderly is 20–40 percent less due to less total body water and lean body weight.29 The alpha (distribution) half-life is 6 hours and is complete in 10 hours.28 Due to lithium following a two-compartment model, serum levels of lithium should be taken only after distribution is complete.
CLEARANCE
Lithium is not metabolized and is almost exclusively eliminated renally via proximal tubule.2 Negligible amounts of lithium are eliminated in the saliva, sweat, and feces. Lithium is filtered via the glomerular membrane and 80 percent of lithium is reabsorbed through the proximal tubule.3 Tubular reabsorption of lithium is closely linked to sodium. Lithium clearance is proportional to the GFR and renal blood flow, and in patients with a normal sodium balance is 25 percent of the creatinine clearance.30 The adult lithium clearance is 0.024 L/hr/kg and is reduced in the elderly to 0.015 L/hr/kg.31
HALF-LIFE
The alpha half-life of lithium is 6 hours, and the beta half-life is 20–24 hours.3,29 The plasma half-life of lithium increases to 48 hours in patients with renal failure. The time to achieve steady state with lithium is 3–5 days. Although lithium concentrations plateau at steady state within 3–5 days allowing for precise dosing adjustments in order to achieve target lithium serum levels, clinicians should be cognizant that the clinical effects of lithium may take up to 14–21 days.
LITHIUM BLOOD SAMPLING
Because lithium distribution follows a two-compartment model, lithium plasma levels need to be sampled after equilibrium between the first and second compartments is complete. This process generally takes 8–12 hours after the last dose.28,29,32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


