The lamina propria of the larynx is composed of a loose stromal tissue with numerous small vessels, lymphatic channels, and nerves. The vocal cord is an exception, and although some small capillaries are seen here, few, if any, lymphatic channels can be found (1). A few scattered inflammatory cells, including mast cells, are sometimes seen. Adipose tissue is intermingled with the lamina propria stroma and the skeletal muscle of the larynx. Two pairs of paraganglia are normally present within the larynx (7). The superior paraganglia are supraglottic and located within the false cords, whereas the inferior paraganglia are usually found near the cricoid cartilage.
The laryngeal framework is then composed of the laryngeal cartilages, ligaments, and intrinsic and extrinsic muscles (1). The cartilages include the thyroid cartilage, a shield-shaped structure that forms most of the anterior surface of the larynx; the cricoid cartilage, the only complete ring of cartilage in the tracheobronchial tract that forms most of the posterior support of the larynx; the paired arytenoid cartilages, the mobile cartilages that articulate with the cricoid cartilage allowing for phonation; and the epiglottis. The epiglottis is elastic cartilage, whereas the others are hyaline cartilages that ossify as individuals grow older.
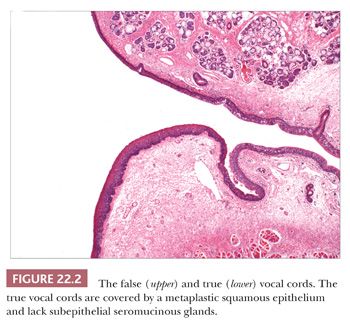
The most prominent ligament is the vocal cord ligament, which runs just over the intrinsic muscle of the true cord (the thyroarytenoid muscle) and connects the thyroid and arytenoid cartilages. It appears as a white band by laryngoscopy and can frequently be seen in biopsies of the vocal cords lying immediately beneath the sparsely vascular submucosal stromal tissue (Reinke space). Other dense ligaments anchor the thyroid and cricoid cartilages together and tether the arytenoids to the cricoid cartilage. Between the various cartilages, joint spaces lined by synovium are present.
The muscles of the larynx may be either extrinsic, those connecting the larynx to other structures, or intrinsic. Extrinsic muscles include the omohyoid and sternohyoid muscles (the hyoid bone, although not actually a portion of the larynx, is connected to it by ligaments) and the sternothyroid and thyrohyoid muscles. Intrinsic muscles include the cricothyroid, posterior and lateral cricoarytenoids, and the thyroarytenoids. The extrinsic muscles of the larynx help to move it primarily during swallowing, whereas the intrinsic muscles function primarily for vocalization.
The superior and inferior laryngeal arteries supply the larynx (1). The superior laryngeal artery and cricothyroid arteries are branches of the superior thyroid artery, which arises from the external carotid. The inferior laryngeal artery is a branch of the inferior thyroid artery, which arises from the thyrocervical trunk of the subclavian artery. The arteries are accompanied by the superior and inferior laryngeal veins, which drain into the superior and inferior thyroid veins, respectively. The lymphatic system then drains along the vascular system of the larynx, with the supraglottic larynx draining superiorly and the subglottic larynx draining inferiorly. The superior laryngeal lymphatic system then empties into jugulodigastric and midjugular nodes, whereas the inferior laryngeal lymphatic system empties into the pretracheal, paratracheal, and inferior jugular nodes.
NONNEOPLASTIC LESIONS OF THE LARYNX
The larynx is most often biopsied because of suspicion of malignancy. Many biopsies will be diagnosed as conventional squamous cell carcinomas; however, numerous nonneoplastic pathologies also involve the larynx.
METAPLASIA AND HYPERPLASIA
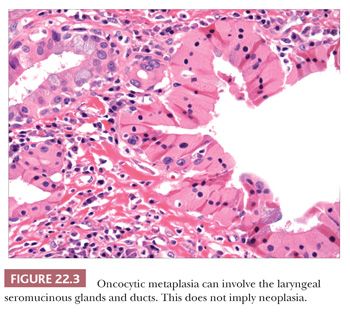
Several metaplastic lesions occur within the larynx that clinically mimic malignancy. Necrotizing sialometaplasia is a metaplastic condition of the seromucinous glands that may develop spontaneously or secondary to injury, often iatrogenic (8–10). Characteristically, the seromucinous glands show a mixture of necrosis and squamous metaplasia. Cytologic atypia is sometimes present, especially in lesions developing after radiation therapy. Unlike squamous cell carcinoma, necrotizing sialometaplasia usually retains a lobular architecture, and admixed normal seromucinous glands are usually present. Chondroid metaplasia of the vocal cord is common, usually affecting the mid or posterior portions of the cord (11,12). The lesions are typically surrounded by dense elastic tissue that blends into the surrounding stroma and do not have the lobular growth pattern typical of chondroid neoplasms. Oncocytic metaplasia of the seromucinous glands is also common and does not imply salivary gland–type neoplasia (Fig. 22.3) (13,14). It is often seen with nonneoplastic laryngeal cysts (discussed later).
Pseudoepitheliomatous hyperplasia is a reactive hyperplasia of squamous epithelium that can occur throughout the body (15). It may be associated with wide variety of neoplastic or inflammatory conditions but is especially noted with granular cell tumors (16,17). Histologically, it is characterized by downgrowths of a thickened squamous epithelium into the underlying stroma, generally with quite elongated and jagged rete. Although some mild architectural and cytologic disturbance may be present with focal abrupt keratinization and mild cytologic atypia, individual cell dyskeratosis and marked cytologic atypia should not be seen.
CONTACT ULCER
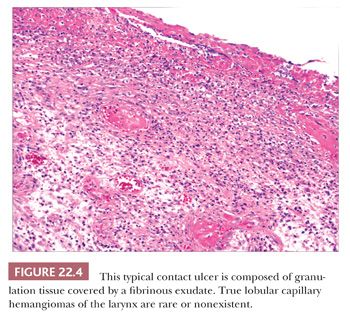
Contact ulcer or laryngeal granuloma is a relatively common lesion that typically involves the posterior portion of one or both sides of the glottis (18–23). Most lesions are traumatic and develop secondary to many causes including, but not limited to, endotracheal intubation, acid reflux (peptic contact ulcer), and vocal cord abuse related to shouting, coughing, and so on. Patients tend to be older than 30 years of age, and most are men. They present with hoarseness, low-pitched voices, frequent coughing, and even severe pain. The posterior vocal cords are usually ulcerated; however, some lesions can have a marked nodular or polypoid appearance simulating neoplasia. Although the epithelium is frequently ulcerated and covered with a fibrinous exudate, lesions can be covered by a proliferative epithelium that may resemble pseudoepitheliomatous hyperplasia. Abundant granulation tissue is seen either at the ulcer site or immediately beneath the squamous epithelium and comprises the bulk of these lesions (Fig. 22.4). Occasional atypical stromal cells are sometimes seen; however, stromal mitotic figures are rare.
VOCAL CORD POLYPS AND NODULES
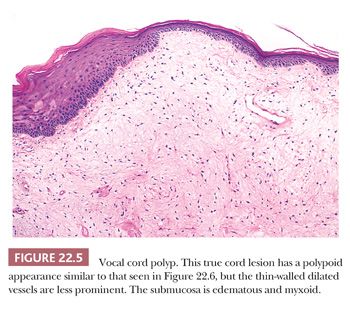
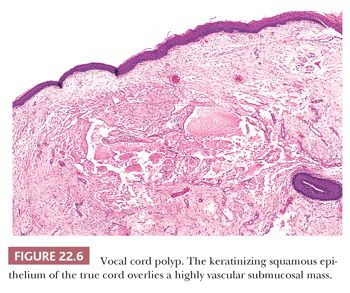
Vocal cord polyps or singer’s nodules occur with equal frequency in men and women and can be found at any age (some have noted the lesions to be more common in men) (24–26). The patients present with hoarseness frequently after abusing their voices (overly enthusiastic bowlers appear to have been especially at risk). The lesions are usually unilateral, located on the anterior third of the vocal cord, and less than 5 mm in size. Histologically, the overlying squamous epithelium is most often intact and is sometimes hyperplastic or, conversely, atrophic. The underlying stroma is paucicellular because of edema or, less commonly, fibrin deposition. Rarely, the stroma may appear hyaline or myxoid (Fig. 22.5). Dilated vessels are frequently seen with a mild amount of chronic inflammation and hemosiderin-laden macrophages (Fig. 22.6). Occasional cases have been noted to have atypical stromal cells.
Vocal cord nodules are similar histologically to vocal cord polyps; however, they tend to be bilateral and occur slightly more posteriorly at the border of the anterior and middle third of the vocal cord (24). Reinke edema may be either unilateral or bilateral and usually is described as a sessile swelling. It also shares the histologic features typical of vocal cord polyps.
NONNEOPLASTIC CYSTS
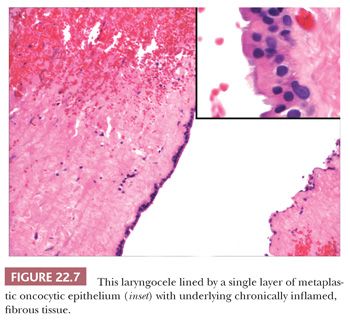
Nonneoplastic cysts of the larynx include laryngoceles, saccular cysts, and ductal cysts (27–30). Although laryngoceles and saccular cysts have a similar histology, they occur in slightly different clinical situations. Laryngoceles are cystic dilatations of the saccule of Morgani, communicate with the larynx, and are air filled. Those that are “internal” are limited to the larynx. These extend from the saccule posterosuperiorly into the area of the false cord and aryepiglottic fold. “External” laryngoceles extend through the opening in the thyrohyoid membrane into the soft tissues of the neck. Laryngoceles are less common than saccular cysts and develop in either sex at any age. Patients present with hoarseness or cough and, when the lesion extends into the neck, a mass. Histologically, laryngoceles are lined by a columnar, ciliated epithelium and may be surrounded by chronic inflammation. Focally, the epithelium can appear more oncocytic (Fig. 22.7) or squamous.
Saccular cysts also occur at the laryngeal saccule; however, they do not communicate with the larynx and are filled with mucin (28,29). Saccular cysts are either lateral and extend posterosuperiorly into the false cord and aeroepiglottic fold or even through the thyrohyoid membrane or are anterior and extend medially and posteriorly and thus protrude into the laryngeal lumen. Like laryngoceles, saccular cysts can occur at any age and may even be congenital. The cysts are generally lined by a ciliated, columnar epithelium that may have squamous or oncocytic metaplasia.
Ductal cysts occur throughout the larynx and are thought to result from the retention of mucus within the ducts of the submucosal seromucinous glands (28,30). These cysts are much more common than saccular cysts and are lined by a columnar or cuboidal mucinous epithelium that can have squamous or oncocytic metaplasia. Because these frequently involve the supraglottic larynx, they are occasionally associated with tonsillar tissue.
FOREIGN BODY REACTION
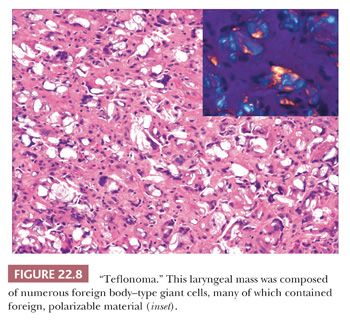
Foreign body reactions within the larynx are often iatrogenic (22,31–34). The most extensively studied reaction is that secondary to Teflon injection used to restore glottic competence. A resultant foreign body reaction may form a mass that bulges into the larynx. Histologically, clusters and sheets of multinucleated foreign body–type giant cells containing birefringent clear material are present with intermixed chronic inflammation and fibrosis (Fig. 22.8). Other material, including silicone, Gore-Tex, and titanium, may be used for vocal cord medialization and can result in similar changes.
SARCOIDOSIS
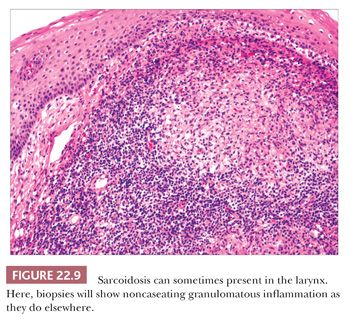
Between 1% and 5% of patients with sarcoidosis will have symptomatic involvement of their larynges (35–37). Clinically, the supraglottic larynx is most frequently affected, and the lesions may appear edematous, granular, or even nodular. The histologic features are classic for sarcoid with numerous noncaseating granulomata with occasional Schaumann or asteroid bodies (Fig. 22.9).
AUTOIMMUNE DISEASE
Systemic autoimmune disease such as rheumatoid arthritis and systemic lupus erythematosus can involve larynx, as can autoimmune disease that primarily affects mucosa and skin. Although involvement of the larynx usually only occurs in well-developed disease, it can sometimes represent the initial presentation of the disease. Because such cases are rare, other disease is usually suspected clinically.
Up to one-quarter of patients with rheumatoid arthritis have involvement of the larynx, and a characteristic synovitis can be demonstrated in biopsy specimens of the cricothyroid and cricoarytenoid joints (38). Rheumatoid nodules may involve the larynx, and it is these lesions that are most likely to be sampled by biopsy (39). These have the typical appearance of necrobiotic collagen nodules with central fibrinoid necrosis surrounded by palisading histiocytes with some lymphocytes and occasional multinucleated giant cells.
Wegener granulomatosis involves the upper or lower respiratory tract, and laryngeal disease occurs in up to 25% of patients affected (22,37,40,41). Here, it may progress to subglottic stenosis. The histologic features are similar to those seen elsewhere. Although necrosis, vasculitis, and granulomata are considered diagnostic of the disease, the three are seldom seen together in biopsy specimens. In fact, many cases will show only nonspecific acute and chronic inflammation.
Relapsing polychondritis is a rheumatic disease characterized by episodic and progressive inflammation of cartilage (42,43). It may involve multiple structures throughout the body but most frequently affects the external ear, joints, eye, respiratory tract, nose, and inner ear. Involvement of laryngeal cartilages and the epiglottis can lead to severe airway compromise as the cartilaginous support of the airway fails. The diagnosis of relapsing polychondritis is generally based on clinical criteria; however, histologic findings are also often a requirement. Histologically, the cartilage usually shows a loss of basophilia or metachromasia with perichondrial acute and chronic inflammation. Cartilage destruction and its replacement by fibrous tissue are also seen.
Several other autoimmune diseases affect the larynx. Systemic lupus erythematosus can be associated with laryngeal rheumatoid nodules (44). Diffuse acute and chronic inflammation may also be seen with hematoxylin bodies and lupus erythematosus (LE) cells, and although less common, a vasculitis with fibrinoid necrosis may also be seen. Sjögren syndrome may also involve the larynx and, as with lupus, can be associated with rheumatoid nodules (45,46). The disease may also be associated with more conventional-appearing vocal cord polyps possibly secondary to a lack of secretions. An intense infiltrate composed of lymphocytes and plasma cells can also be seen with the disease. Bullous pemphigoid and pemphigus vulgaris may both involve the larynx (47,48). The histologic features are similar to those seen elsewhere. Finally, Crohn disease rarely involves the upper aerodigestive tract and larynx (49). It is histologically characterized by nonspecific chronic inflammation; rarely, noncaseating granulomata may be seen.
DEPOSITION AND OTHER BENIGN DISEASE
Patients with long-term hyperuricemia eventually develop soft tissue deposits of crystallized uric acid (gout). Rarely, these have been reported in the larynx (50–52). Patients have typically complained of hoarseness and dysphagia and are noted clinically to have mass lesions, often involving the cords, that limit the mobility of the cords. Histologically, pseudoepitheliomatous hyperplasia may be present overlying deposits of an amorphous negatively birefringent crystalline material that is surrounded by a foreign body–type reaction.
Amyloid deposition within the larynx may be localized or secondary to systemic disease (53–58). In the upper aerodigestive tract, amyloid deposits are sometimes seen with extramedullary plasmacytomas (59). Patients vary widely in age, with the mean age at presentation in the fourth or fifth decades of life. They typically present with progressive hoarseness. The lesions are usually described as supraglottic or transglottic and range from firm, elevated, smooth lesions to polypoid mass-like lesions that have a tan-yellow to red-grey cut surface. Histologically, these lesions characteristically have abundant amorphous, eosinophilic material deposited extracellularly within the lamina propria; prominent periglandular or perivascular deposition is sometimes seen. Scattered lymphocytes and plasma cells are frequently noted with occasional macrophages and rare giant cells. Congo red or methyl violet histochemical staining can be used to confirm the diagnosis of typically light chain amyloid. Light chain restriction can sometimes be demonstrated in the plasma cell population and predicts recurrent disease. The diagnosis should obviously instigate further workup for systemic causes of amyloid deposition.
Eosinophilic angiocentric fibrosis can involve the larynx, and rare cases have been reported to cause breathing difficulties (60). Like the cases in the nasal cavities or paranasal sinuses, these lesions have abundant fibrosis that appears to wrap around small blood vessels. Numerous eosinophils are seen with occasional lymphocytes and plasma cells. It is now believed that this disease may fall within the spectrum of IgG4-related disease and thus immunohistochemical staining of IgG4 and knowledge of serum IgG4 concentration may be helpful for diagnosis (60a).
The larynx may be involved by tracheopathia osteoplastica, a bizarre condition that most commonly affects men over the age of 50 years (61,62). Patients may present with hemoptysis, cough, hoarseness, or wheezing. The mucosa will appear cobblestone as hard, irregular nodules project into the laryngeal and tracheal lumina. Histologically, islands of hyaline cartilage and lamellar bone are present immediately beneath a metaplastic squamous epithelium.
Subglottic laryngeal stenosis is most often acquired; however, occasional cases may be congenital (63–65). Causes include trauma, prolonged intubation, burns, surgery, radiotherapy, infection, autoimmune disease, neoplasia, sarcoid, amyloidosis, and, possibly, gastroesophageal reflux. Patients usually have difficulty breathing, and children may be noted to have feeding abnormalities or abnormal crying. The larynx will appear markedly narrowed, sometimes by an apparent mass lesion. Histologically, sections usually show a nonspecific fibrosis with dense eosinophilic collagen, fibroblasts, and variable chronic inflammatory infiltrate. Clinical history may be helpful for determining the etiology of the disease.
Hamartomas of the larynx are extremely rare (66). They appear somewhat reminiscent of respiratory epithelial adenomatous hamartomas of the nasal cavity with a disorganized proliferation of respiratory epithelium, seromucinous glands, smooth muscle, and cartilage.
INFECTIOUS DISEASE
The larynx may be infected by a great host of organisms that more commonly involve the lungs or sinonasal area. Many of these entities present as mass lesions, and an infectious etiology is often unsuspected. Pathologists, however, should be well aware of these lesions because the correct diagnosis can spare a patient unnecessary treatment.
Mycobacterial Infections
Since the advent of effective antituberculosis treatments, laryngeal involvement by tuberculosis has only seldom been reported, and when reported, it has been described as a clinical and gross mimic of laryngeal carcinoma (37,67–70). Laryngeal disease shares the histologic features of tuberculosis found elsewhere, with caseating granulomatous inflammation and Langhans giant cells. Histochemical staining for acid-fast bacilli can sometimes be helpful.
The larynx is the second most common site in the head and neck after the sinonasal area to be involved by leprosy (37). Laryngeal leprosy presents with painless ulcerated and nodular lesions located supraglottically that later progress to involve the glottis. Histologically, the process is similar to that noted in the nasal area and is usually composed of a mixture of chronic inflammatory cells and large, foamy macrophages. Mycobacterium leprae (Hansen bacillus) may be identified by histochemical staining for acid-fast bacilli.
Fungal Infections
Fungal infections of the larynx are uncommon, are frequently associated with widespread disease, and are most often found in immunocompromised patients. Laryngeal histoplasmosis may present as either multiple nodular or ulcerated lesions of the vocal cords (37,71). A mixture of acute and chronic inflammation is usually present with numerous large macrophages with abundant granular cytoplasm with or without well-formed granulomata. The overlying epithelium is often ulcerated; however, marked pseudoepitheliomatous hyperplasia may be present and should not be confused with squamous cell carcinoma. Although the organisms can frequently be seen on routine, hematoxylin and eosin (H&E)–stained material, silver or periodic acid-Schiff (PAS) staining also can be used to highlight their presence.
Patients with laryngeal blastomycosis typically present with hoarseness, with or without coexistent pulmonary or cutaneous lesions (37,72,73). It is typically described as erythematous, irregular, and granular lesions that may also appear verrucoid. Histologically, these lesions are characterized by pseudoepitheliomatous hyperplasia with mixed acute and chronic inflammation, Langhans giant cells, intraepithelial microabscesses, and granulomata, some of which may be suppurative. The causative organism, Blastomyces dermatitidis, can usually be identified with H&E-stained material or with silver or PAS stain.
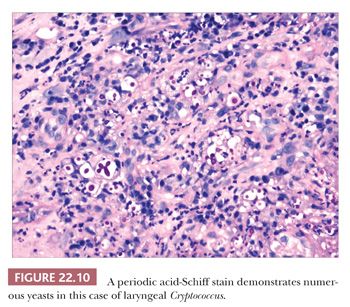
Other fungal organisms may also involve the larynx, and infections secondary to Coccidioides immitis, Cryptococcus neoformans, Candida albicans, Aspergillus species, Rhinosporidium seeberi, and Paracoccidioides have been reported (74–80). Each of these may be seen in immunocompromised patients with or without more widespread disease. Occasionally, they may present in apparently immunocompetent patients without evidence of disease elsewhere. Patients typically present with hoarseness and have granular and sometimes exophytic lesions involving the larynx. As with other fungal infections, pseudoepitheliomatous hyperplasia is often present. A mixed inflammatory infiltrate is typically seen that can involve the epithelium. Fungal organisms may be identified on routine and special stains (Fig. 22.10). A mucicarmine stain can be used to highlight the capsule of C. neoformans.
Bacterial Infections
Bacterial infections of the supraglottic larynx and hypopharynx are now rare thanks to vaccinations for Haemophilus influenzae and Corynebacterium diphtheriae (81,82). Histologically, H. influenzae infection will show abundant acute inflammation. Patients with diphtheria have ulceration of the oral cavity, pharynx, and larynx with pseudomembrane formation. Histologically, the pseudomembranes contain fibrin, neutrophils, cell debris, and bacteria, whereas the underlying stromal tissues are edematous with acute inflammation.
Secondary and tertiary syphilis rarely involves the larynx (37,75). Histologically, secondary syphilis is characterized by a thinned epithelium or epithelial hyperplasia (condyloma latum) with a dense lymphoplasmacytic infiltrate, sometimes with granulomas. Tertiary syphilis is characterized by gummas, which are destructive and painless necrotic lesions. Histologically, these are characterized by necrotizing, granulomatous inflammation. A lymphoplasmacytic vasculitis may be noted in both secondary and tertiary forms of disease. Spirochetes may be identified in earlier lesions with silver stains (Warthin-Starry, Dieterle, etc.).
The larynx is rarely involved by rhinoscleroma secondary to infection by Klebsiella rhinoscleromatis (83). Patients usually also have sinonasal disease; however, isolated laryngeal infection has been reported. The histologic features are similar to those of the sinonasal area. Rare cases of laryngeal infection secondary to Actinomyces have been reported (84). Histologically, these lesions characteristically have sulfur granules (basophilic clusters of bacteria with a peripheral, more eosinophilic zone of radiating filaments) surrounded by acute inflammation and granulation tissue.
SQUAMOUS PAPILLOMA
Squamous papillomas of the larynx are warty growths that are categorized according to their number (solitary or multiple) and the age of the patient affected (juvenile or adult) (85–95). Most laryngeal papillomatosis occurs in younger patients. These lesions usually are located in the region of the true vocal cords; however, they may be found throughout the larynx and throughout the oropharynx, trachea, and even lung. They often recur after resection and may cause obstruction. Rarely, patients with long-term disease develop well-differentiated squamous cell carcinomas, often without other risk factors for laryngeal or pulmonary squamous cell carcinoma (96,97). Some have developed in patients who received radiation therapy for their disease. Most juvenile lesions and many of the adult lesions are etiologically related to infection of the mucosa with human papillomavirus (HPV) types 6 and 11, although some have been associated with HPV types 16 or 18 (86,98). Indeed, rather limited evidence has been used to claim that patients with lesions associated with HPV-16 or 18 are at higher risk for developing subsequent malignancy (99). In cases of juvenile papillomatosis, it is thought that infection occurs at birth, whereas with adults, it is believed that the infection may occur at a later age.
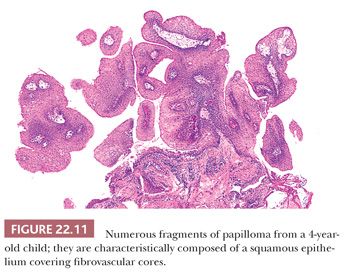
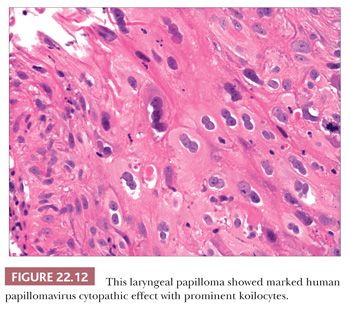
Grossly, the lesions are variably sized but are usually smaller than 1 cm in greatest dimension. Histologically, they are characterized by a branched fibrovascular core covered with maturing stratified squamous epithelium (Fig. 22.11). Keratosis is generally not seen. Koilocytic change is sometimes present; however, intraepithelial dysplasia is infrequent, and severe or full-thickness dysplasia is very uncommon and should suggest a diagnosis of papillary carcinoma (Fig. 22.12).
The differential diagnosis should include other squamoproliferative lesions, especially squamous cell carcinoma, and some cases of laryngeal papillomas in older individuals may be very difficult to separate from papillary squamous cell carcinomas or verrucous carcinomas. Indeed, reported solitary papillomas in adults associated with malignancy likely represent sampling errors rather than progression of disease from a true papilloma. Thus, clinical follow-up and complete excision are recommended for any adult with a solitary papillary lesion that shows any degree of atypia (88,90,93). In general, malignancies are destructive or will show invasion; however, history is often unavailable, and invasion may be difficult to assess with small biopsies. Papillary squamous cell carcinoma should show full-thickness cytologic atypia. Verrucous carcinomas will not, by definition, show severe cytologic atypia but often show keratinization.
PRECURSOR LESIONS OF SQUAMOUS CELL CARCINOMA
As with squamous cell carcinoma of the cervix, squamous cell carcinoma of the larynx develops through progressive, worsening intraepithelial lesions prior to becoming invasive (100–110). Clinically, precursor lesions may appear white or red (or speckled) by endoscopy and are termed leukoplakia and erythroplakia. Although these clinical lesions do not correspond with definitive histologic features, speckled and red lesions (i.e., erythroplakia) are more likely to show squamous dysplasia and be associated with concurrent squamous cell carcinoma or the eventual development of squamous cell carcinoma. The data regarding the risk of these lesions progressing to malignancy are varied, and it is estimated that somewhere between 5% and 20% of clinically apparent intraepithelial lesions will develop into invasive malignancies. Studies that have quantified the amount of intraepithelial dysplasia have found that worsening dysplasia is associated with increased risk.
The World Health Organization (WHO) currently uses a five-tier system for the grading of intraepithelial neoplasia of the larynx and classifies these lesions as hyperplasia, mild dysplasia, moderate dysplasia, severe dysplasia, and carcinoma in situ (105). Although the descriptions of these lesions sound somewhat similar to the progressive abnormalities seen with cervical specimens, these lesions are not histologically identical, and only rarely does laryngeal intraepithelial dysplasia show a progressively basaloid appearance akin to cervical intraepithelial neoplasia. Indeed, many laryngeal squamous cell carcinomas are associated with intraepithelial lesions that do not appear to meet the criteria for carcinoma in situ or even severe dysplasia.
The WHO classification scheme uses two histologic parameters for the diagnosis of squamous precursor lesions of the upper aerodigestive tract—architectural and cytologic atypia. Architectural disturbance is the result of disturbed cellular maturation. This is manifest histologically through lack of typical nuclear polarization; non–basally located mitotic figures; dyskeratosis; and abrupt, early keratinization. Unlike with cervical intraepithelial neoplasia, dyskeratotic cells are very helpful for the diagnosis of squamous intraepithelial neoplasia of the larynx and may be one of the few histologic clues of dysplasia. The identification of drop-shaped rete ridges can also be helpful, especially for the diagnosis of higher grade dysplastic lesions. Atypical cytologic features include anisonucleosis and nuclear pleomorphism, anisocytosis and cytologic pleomorphism, increased nuclear size and increased nuclear-to-cytoplasmic ratios, nuclear hyperchromasia, prominent nucleoli, and atypical mitotic figures. The greater the degree of architectural disturbance and cytologic atypia are, the higher the grade of dysplasia.
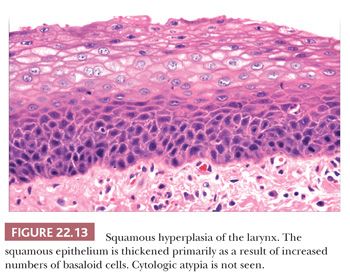
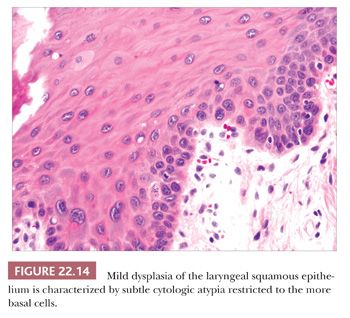
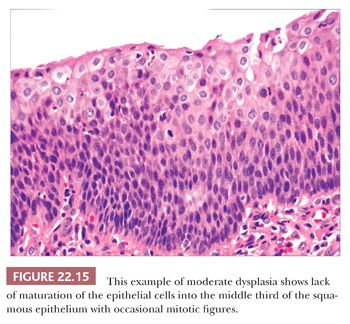
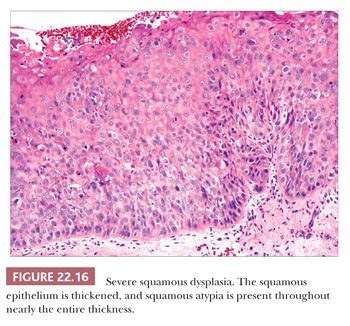
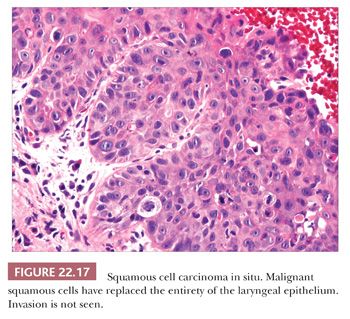
Squamous hyperplasia is characterized by an increase in epithelial thickness (i.e., increased numbers of cells) and, often, keratosis (Fig. 22.13). The cells may be located throughout the epithelium or appear somewhat localized. These lesions should be devoid of architectural or cytologic atypia. Although considered to be a precursor lesion, the risk for the eventual development of squamous cell carcinoma in patients with these lesions is low. Mild dysplasia shows minimal architectural and cytologic atypia. Most of the lesions described as mild dysplasia appear to show some basal cell hyperplasia with cytologic atypia confined to the lower one-third of the epithelium (Fig. 22.14). Moderate dysplasia is characterized by a greater degree of architectural disturbance than mild dysplasia, with architectural changes extending into the middle one-third of the epithelium (Fig. 22.15). However, the atypia may also be more pronounced; if it is severe, such lesions may be categorized as severe dysplasia. Lesions with severe dysplasia have architectural disturbances that extend to more than two-thirds of the thickness of the squamous epithelium with marked cytologic atypia (Fig. 22.16). Finally, carcinoma in situ is defined as malignant transformation without actual invasion. Histologically, full-thickness architectural disturbance should be accompanied by severe cytologic atypia (Fig. 22.17).
Progressive molecular abnormalities have been shown to correlate with progressive histologic atypia and the risk of subsequent invasive squamous cell carcinoma (111,112). Worsening atypia correlates with aneuploidy, which has been shown to correlate with progression (102,113). Loss of heterozygosity (LOH) studies have shown that the losses at 3p and 9p are likely early events in the development of squamous cell carcinoma of the upper aerodigestive tract and that such losses correlate with the risk for malignant transformation (114–116). Furthermore, it has been shown that additional molecular abnormalities increase the risk even more. Much attention has also been paid to p53 mutations, which are common in precursor lesions and are associated with increased risk for the development of invasive disease; however, other tumor suppressor genes, including but not limited to p16, p14, p12, p21, p27, MGMT, and retinoblastoma (RB), have been shown to be mutated or lost by other means to some degree in precursor and malignant lesions of the larynx (114,116–119). Cyclin D1 overexpression has also been noted, as has overexpression of epidermal growth factor receptor (EGFR) (120).
Ancillary studies are usually not very helpful for diagnosing precursor lesions, likely because of the heterogeneous molecular events that can lead to squamous cell carcinoma of the larynx. Most reports have used immunohistochemistry with antibodies directed toward proteins that are lost or gained secondary to genetic alterations such as those just mentioned. Immunostaining for p53 protein has often been touted as useful, as mutations of the gene are frequent and generally lead to an accumulation of defective protein (121). Proliferation markers such as Ki-67 may also be helpful, and substantial nuclear labeling above the basal layer of the epithelium may be associated with intraepithelial neoplasia (122). Immunostaining for the p21 protein, a target gene that can be activated by p53, may also be helpful, although results have been mixed. Although some authors have attempted to use the expression of various keratins to assist in the diagnosis, the results have proven to be too mixed or inconsistent to be of much use (121).
Verrucous hyperplasia is a term used to describe a verrucous and keratotic form of squamous hyperplasia that can show varying degrees of cytologic atypia (123,124). These lesions are more aggressive than conventional forms of intraepithelial neoplasia and are more likely to progress to malignancy. Histologically, they are characterized by folded, keratotic, hyperplastic squamous epithelium. Unlike verrucous carcinomas, these lesions are not yet invasive, and the bases of the lesions should not appear deeper than the surrounding normal squamous epithelium. Furthermore, although often without cytologic atypia, these lesions may show cytologic atypia, which should not be seen with verrucous carcinoma.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma accounts for the vast majority of laryngeal malignancies. It was predicted that a little more than 12,000 people would be diagnosed with laryngeal cancer in the United States in 2013 and that more than 3600 people would die from the disease (125). This equates to an age-adjusted incidence rate of 3.4 per 100,000 people per year and an age-adjusted mortality rate of 1.1 per 100,000 people per year. Less than 5% of patients diagnosed are less than 45 years of age, and cancer of the larynx is approximately five times more common in men.
Laryngeal squamous cell carcinoma is a smoking-related disease, and it is estimated that, with smoking, there is a 10-fold increased relative risk for laryngeal carcinoma (126–130). Alcohol consumption, as with other head and neck carcinomas, also plays a role because patients who drink heavily and smoke are at a much increased risk for the development of laryngeal squamous cell carcinoma. The risk of other possible factors in the development of this disease, including oncogenic viruses (including HPV), asbestos exposure, familial susceptibilities, and gastroesophageal reflux, remains unclear, but it is likely trivial (131–133). With the decline of per capita cigarette consumption in the United States since the 1960s, the incidence of laryngeal cancer has also declined, although the decline in disease incidence trails by approximately 15 years (134–136). Mortality rates have also followed suit, roughly mirroring the changes in incidence. Indeed, the incidence and mortality rates for laryngeal cancer in the United States are now roughly 70% of what they were in 1975. These changes suggest that current multimodality therapies have done little to change the mortality rate of laryngeal squamous cell carcinoma. Furthermore, the 5-year survival rates for the disease have changed little and even slightly worsened over the past 20 years.
Staging parameters have, in general, been developed to predict outcome. As with cancer at many sites, the localization of a laryngeal cancer plays a key role in its staging and outcome. According to the National Cancer Institute (NCI), approximately 56% of laryngeal squamous cell carcinomas are localized at the time of diagnosis, 20% have metastasized to lymph nodes or directly spread from their primary site, and 18% have metastasized distally (125); the corresponding 5-year relative survival rates are 76%, 42%, and 35%, respectively.
The current American Joint Committee on Cancer (AJCC) staging system for laryngeal squamous cell carcinoma subdivides the tumors based on their relationship to the glottis (137). The node (N) and metastasis (M) statuses throughout the larynx are determined using the same criteria. N1 metastases are those confined to a single ipsilateral lymph node and 3 cm or less in size. N2a metastases are those confined to a single ipsilateral lymph node and between 3 and 6 cm in size. N2b disease is defined by the presence of multiple ipsilateral lymph node metastases, all less than 6 cm in size. N2c disease is defined as metastases to bilateral or contralateral nodes, all less than 6 cm in size. N3 metastases are those lymph node metastases that are greater than 6 cm in greatest dimension. All distant metastases are simply classified as M1.
The tumor (T) portion of the staging parameters for laryngeal carcinoma varies depending on the site of the primary tumor (137). This reflects the anatomy of the larynx, which in turn likely influences the behavior of laryngeal tumors. Examples of this include the frequent involvement of the preepiglottic space by supraglottic carcinomas likely secondary to the fenestrations that pass through the epiglottis. Another example includes the infrequent metastasis of small glottic tumors, perhaps secondary to the relative lack of lymphatics at the site.
Because the glottis is a relatively small structure, tumors can sometimes involve the glottis and either or both the supraglottic and subglottic larynx. This is especially reflected in the cases that now come to laryngectomy, as smaller glottic tumors are frequently treated with radiation alone. Tumors should be categorized based on the location of the bulk of the tumor with the caveats that many transglottic tumors should be considered glottic and those tumors extending more than 1 cm below the glottis should be categorized as subglottic. Fortunately, the definitions of the higher T categories are rather similar, regardless of the subclassification of the tumor. The AJCC cancer staging atlas well illustrates the various T levels and can be very helpful when one is staging a laryngeal squamous cell carcinoma (138). Because the T component includes a clinical aspect, it can be relatively confusing for pathologists, and some cases can even be staged pathologically differently from the way they had been staged clinically. With difficult cases, it is incumbent upon the pathologist to ascertain necessary clinical information, usually the status of vocal cord mobility.
Patients present with clinical symptoms related to the site of laryngeal involvement by tumor. Those glottic tumors or subglottic/supraglottic tumors involving the glottis often present with hoarseness. Patients with supraglottic tumors can present with difficulty swallowing, sore throats, referred pain to their ears, and lymph node metastases. Those with subglottic tumors sometimes present with difficulty breathing or metastases.
The treatment of laryngeal squamous cell carcinoma varies depending on the site involved and the stage. Although current treatments seem not to have improved survival rates, they are associated with much less morbidity than previous treatments. Localized, smaller tumors, especially T1 or T2 malignancies, can frequently be treated with radiation alone or, sometimes, laser excision, with more extensive surgery reserved for patients who fail the initial therapy (139,140). Higher stage malignancies require partial or total laryngectomy with subsequent radiation therapy (141,142). Conversely, some patients may receive radiation therapy and even chemotherapy, with surgery used as a salvage therapy (143,144). Patients with laryngeal cancer are frequently also treated with isotretinoin to reduce the up to 25% risk for the development of a second primary malignancy (145).
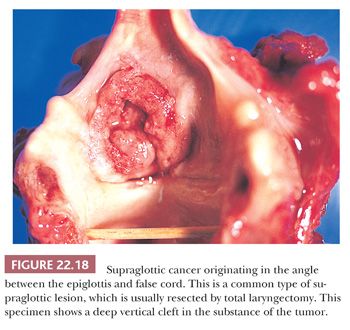
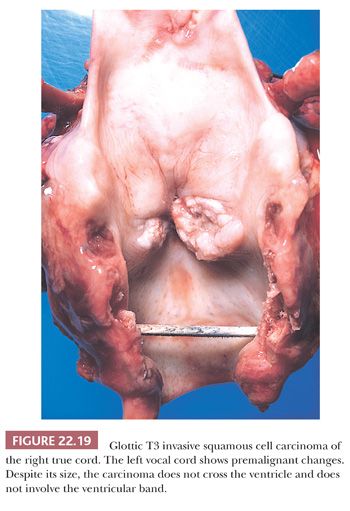
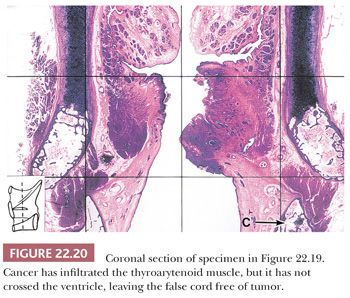
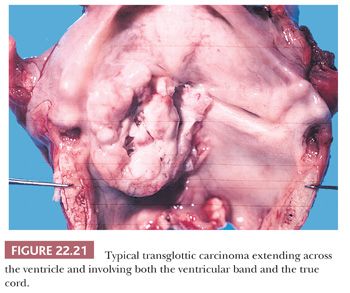
Grossly, squamous cell carcinomas of the larynx are almost always described as fungating, white-tan masses, often with ulceration (Figs. 22.18 to 22.21). Rarely, the lesions appear flat. Other than with small, poorly oriented superficial biopsies, the diagnosis of laryngeal conventional squamous cell carcinoma rarely poses any difficulty for pathologists. The tumors are composed of infiltrative, irregular nests of squamous cells that show varying degrees of intracellular and extracellular keratin formation. With better differentiated tumors, increased differentiation is usually present toward the center of the nests, with the most peripheral cells having a basaloid phenotype and the more central cells having more abundant eosinophilic cytoplasm. Extracellular keratin, commonly intermixed with necrotic debris, is often identified at the centers of the nests (Fig. 22.22). The squamous nests can vary considerably in size, with some appearing large and cystic. Single-cell infiltration may be present, especially with higher grade tumors (Fig. 22.23). The surrounding stroma usually has some degree of chronic inflammation, often with stromal desmoplasia. The nuclei of the neoplastic cells show moderate to marked differences in shape and size with irregular contours and vesicular to granular, malignant-appearing chromatin. Prominent nucleoli are usually seen, and mitotic figures, including atypical mitotic figures, can usually be found. Necrosis of both individual tumor cells and of large portions of tumor is frequently seen with laryngeal squamous cell carcinomas.
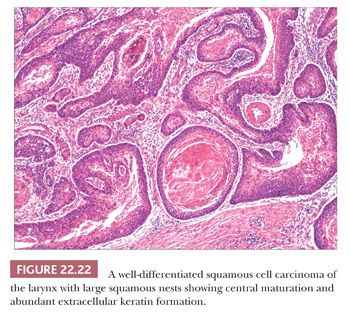
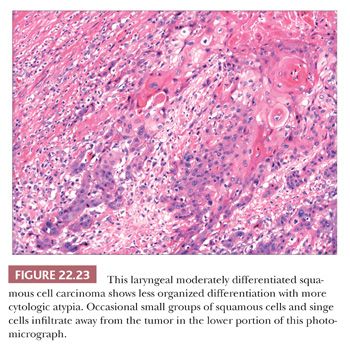
Several histologic parameters are generally reported with cases of laryngeal squamous cell carcinomas that have been shown to have variable prognostic significance. These generally include grade (sometimes recorded from the advancing tumor front), the presence or absence of perineural and angiolymphatic invasion, and margin status (146–150). (Regardless of the fact that most pathologists do not use “standardized” and “objective” criteria for grading, grading frequently conforms to such stated criteria.) Some have suggested that tumor border configuration and degree and character of the inflammatory infiltrate may also be important, as well as the presence of extracapsular extension seen with lymph node metastases (148,151,152). Although not a histologic parameter per se, a measurement of tumor depth of invasion may also provide prognostic information (153).
There are no specific immunohistochemical markers of laryngeal squamous cell carcinomas, and these tumors react with antibodies akin to squamous cell carcinomas from other sites. Conventional squamous cell carcinomas are invariably reactive with antibodies to cytokeratins, with better differentiated tumors expressing higher weight keratins and poorly differentiated tumors expressing lower molecular weight keratins. Immunostaining with antibodies to p63 can also be helpful for the diagnosis of these lesions and can assist the pathologist in distinguishing some tumors with other lesions. However, salivary gland–type tumors frequently also express the antigen.
Cytogenetic and molecular abnormalities associated with laryngeal squamous cell carcinomas have been shown by some to have prognostic value and have been used by some to assist in the diagnosis of difficult biopsies. The findings are similar, although somewhat more advanced, than those seen in preinvasive disease (see previous discussions). Cytogenetically, laryngeal squamous cell carcinomas frequently show gains of 3q, 5p, 8q, 11q13, and 17q with losses of 3p (154,155). p53 and p16 genes are inactivated in some cases of laryngeal squamous cell carcinomas, as is RB, although in a smaller percentage of cases (114). EGFR is amplified in up to 25% of cases (120). The prognostic information that can be gained from studying cytogenetic or molecular markers and even ploidy remains controversial (156,157). Such abnormalities are not frequently investigated or reported in general practice.
VARIANTS OF SQUAMOUS CELL CARCINOMA
All common variants of squamous cell carcinoma have been reported to involve the larynx. These include verrucous carcinoma, sarcomatoid or spindle cell carcinoma, basaloid squamous cell carcinoma, adenosquamous carcinoma, papillary squamous cell carcinoma, adenoid squamous cell carcinoma, and undifferentiated carcinomas. Distinguishing these malignancies from conventional squamous cell carcinomas and other malignancies is important for several reasons that will be discussed with each particular variant.
VERRUCOUS CARCINOMA
The larynx is the second most common site within the upper aerodigestive tract after the mouth to be involved by verrucous carcinoma (158–163). Here, these lesions typically involve the vocal cords and are much more common in men, usually coming to attention in the sixth and seventh decades of life. Most patients present with hoarseness. As with conventional squamous cell carcinomas, these tumors arise most frequently in patients who have abused alcohol and tobacco products. Although numerous studies have been published regarding the etiologic role of HPV with these lesions, its role, if any, is still debated (117,164–166). p53 protein accumulation has been demonstrated by immunohistochemistry with increased proliferative activity (117,167). LOH studies have shown fewer abnormalities with these tumors compared with other more poorly differentiated squamous cell carcinomas, and deletions of 9p have been noted (168).
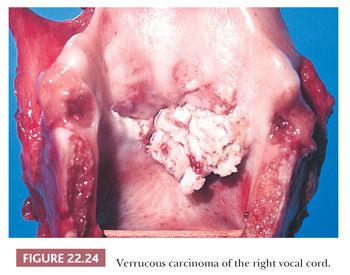
Grossly, the tumors are white and papillary or fungating (Fig. 22.24) (158–160,163,169). The size of the lesions can vary, and tumors can become very large with extensive invasion and destruction of the laryngeal tissues. Histologically, the tumors are composed of a thickened and folded squamous epithelium frequently with abundant keratosis and parakeratosis extending from the filiform peaks of the surface (Fig. 22.25). The tumor bases are circumscribed and composed of broad tongues of squamous epithelium that extend deeper than the surrounding nonneoplastic epithelium into the subepithelial tissues (Fig. 22.26). Invasive, irregular nests of squamous cells should not be seen. Chronic inflammation and, rarely, foreign body–type giant cells are present in the surrounding stroma. Cytologically, little, if any, atypia should be present, with normal maturation and mitotic activity limited to the basal levels of the neoplasm. Occasional dyskeratotic cells are sometimes seen within the epithelium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


