Maximal stress and strain are exerted at the tidemark, where there is an abrupt change in the mechanical properties of the cartilage, the noncalcified malleable surface becoming sharply rigid in this region. Abnormal tensile strain promotes chondrocyte proliferation, maturation, and hypertrophy similar to that seen in the epiphyseal plate, with increased production of collagen X by the chondrocytes. Stimulation of the subchondral bone results in collagen type I production, and ultimately vascular invasion and matrix degradation occur with ossification. Chondrocytes share a common mesenchymal progenitor cell origin with macrophages and may behave as activated macrophages. Like all mesenchymal cells, chondrocytes exhibit plasticity of differentiation; cartilaginous repair is exemplified by increased collagen production with transformation to cells that have a more fibroblastic phenotype, essentially exhibiting the features of fibrocartilaginous metaplasia. In contrast to hyaline cartilage, fibrocartilage has suboptimal biomechanics in terms of maintenance of joint function (2).
Normal synovium is composed of a surface intima/synoviocyte layer comprising one or two layers of lining cells. Some of these are of macrophage origin (expressing CD68), whereas others have a fibroblast-like appearance, and intermediate forms are present. These cells secrete hyaluronic acid, phagocytize waste material from the joint space, and help regulate the metabolic function of the synovial fluid. In the subintimal fibrovascular tissue, the fat content is variable from individual to individual and from site to site.
GENERAL FEATURES OF THE ARTHRITIC JOINT
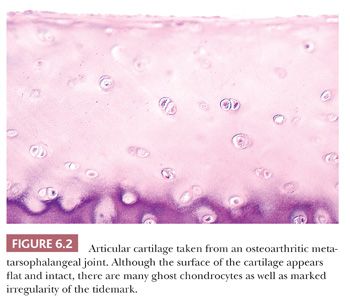
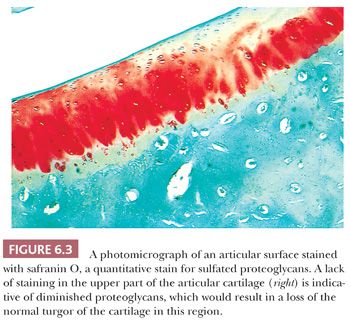
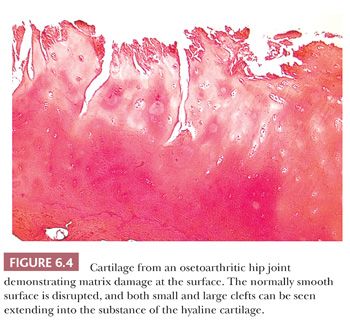
The alterations observed in the tissues of an arthritic joint may be divided into two categories: those that are evidence of injury and those that can be regarded as reparative or reactive (3). These are often seen concurrently. An obvious sign of injury in the cellular component of the cartilage is necrosis of a significant number of chondrocytes (Fig. 6.2). On occasion, complete necrosis of the chondrocytes may be observed, and depletion of proteoglycan in the cartilage matrix is seen as a diminution in basophilic staining in sections stained with hematoxylin and eosin (H&E). This may be highlighted by the use of special stains for proteoglycans such as safranin O or Alcian blue (Fig. 6.3). Collagen injury is recognized by vertical and horizontal clefts within the cartilage matrix (Fig. 6.4) (4). A significant feature of damaged articular cartilage is an irregular, thickened, granular tidemark, indicating disturbed calcification, which is frequently associated with duplication of the tidemark.
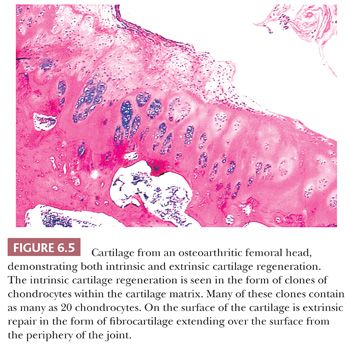
Cartilage regeneration or repair usually presents either as a proliferation of chondrocytes within the damaged cartilage (“cloned” chondrocytes) or as a proliferation of new cartilage cells from the underlying bone and from the periphery of the joint (Fig. 6.5). The proliferative cartilage is likely to be more cellular than normal cartilage; demonstrates increased proteoglycan synthesis; and, on polarized light microscopy, has a different organization of the collagenous component of the extracellular matrix, the collagen being more easily identifiable, overall representing regions of fibrocartilaginous metaplasia (Fig. 6.6).

In the adjacent subchondral bone, injury is evidenced by superficial bone necrosis, microfractures within the superficial bone trabeculae, and the development of localized areas of bone lysis, microscopically characterized by replacement of bone tissue by fibromyxomatous tissue, which may on occasion form cystic spaces.
Reparative changes in the bone are seen as an increase in osteoblastic activity in the subarticular region with new bone formation and thickening of the subchondral trabeculae, representing sclerosis. Remodelling of the articular surface is commonly seen, particularly at the periphery of the joint, where, through the process of endochondral ossification, proliferating cartilage is transformed into bony spurs covered by cartilage or osteophytes, which serve to redistribute the load over the articulating surfaces (5). The cartilage thus formed always has more obvious collagen fibers on polarization, again representing fibrocartilaginous metaplasia.
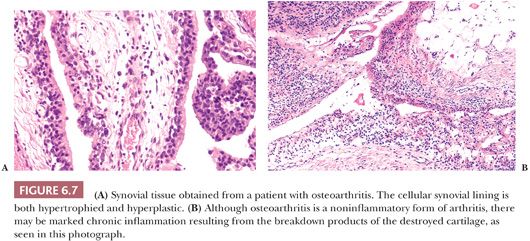
The synovial lining of an arthritic joint usually shows hyperplasia or hypertrophy of the synoviocytes as well as varying degrees of chronic inflammation (Fig. 6.7). Because the hyperplastic synovium of an arthritic joint becomes traumatized in the course of use, some degree of hemosiderin deposition is common, and increased proteoglycan content representing reactive mucoid change may be present (see later discussion). In those diseases in which rapid breakdown of the joint surface occurs (e.g., Charcot joint and rapidly destructive osteoarthritis), pieces of bone and cartilage may be found within the synovial membrane, surrounded by histiocytes and chronic inflammatory cells, so-called detritic debris. This debris may then undergo active resorption by the synovium.
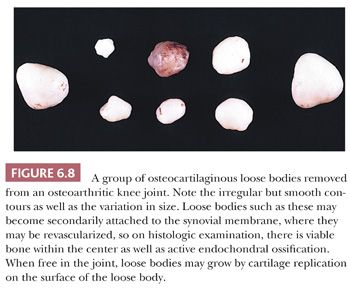
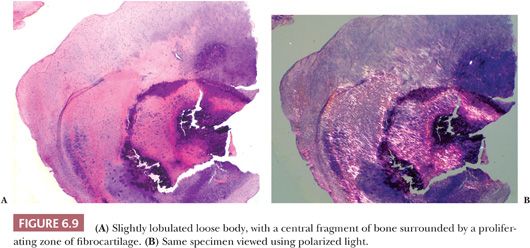
Large pieces of detached cartilage or cartilage and bone within the joint space (loose bodies) may increase in size because of cellular proliferation on their surface, and, as they steadily enlarge, the center of these loose bodies becomes necrotic and calcified (Fig. 6.8). In a resected loose body, it may be possible to see concentric rings of fibrocartilaginous metaplasia with calcification, indicating the history of its growth, similar to the rings in a tree. The initiating tissue may be identifiable centrally (Fig. 6.9). Sometimes the loose bodies secondarily become attached to the synovium and are invaded by blood vessels. Through the process of endochondral ossification, these attached and revascularized loose bodies may become converted to nodules of viable bone within the synovium. It is important to distinguish these secondary posttraumatic osteochondral loose bodies from primary synovial chondromatosis, as discussed later in this chapter.
OSTEOARTHRITIS
In many cases, osteoarthritis arises as a result of mechanical dysfunction after trauma. In recent years, hip osteoarthritis has been increasingly attributed to abnormal shape of the femoral head, resulting in femoroacetabular impingement early in life (6). However, in general, the onset of clinical disease in osteoarthritis does not occur until late middle age. Clinically, the most common presenting joints are the first metatarsophalangeal, lumbar spine, knee, and hip. Although more than one joint may be affected, the disease usually is limited to either one joint or the same joint bilaterally.
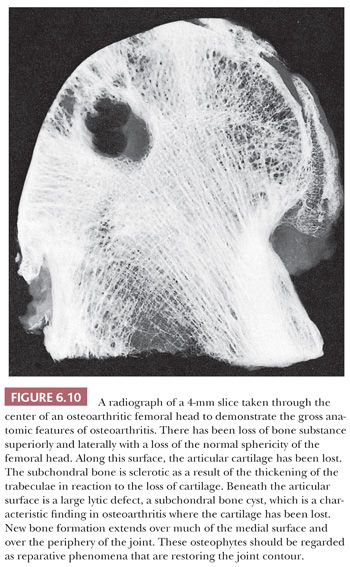
An x-ray image generally shows deformity, loss of the joint space, osteophyte formation, sclerosis of the subchondral bone, and often migration of the joint (Fig. 6.10). Frequently, lucent “cystic” lesions can be seen in the subchondral bone, particularly in the case of arthritis of the hip.
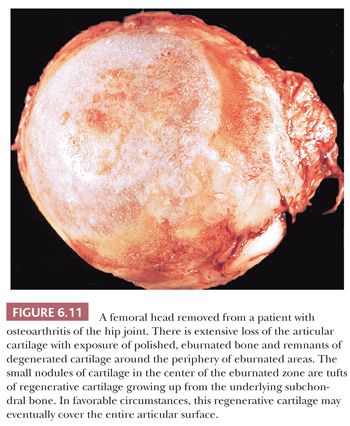
The most significant macroscopic features of an osteoarthritic joint are damage to the articular cartilage, bone loss, and an alteration in the shape of the articulating surface. In large portions of the joint, usually the weight-bearing component, the cartilage may be entirely absent. The exposed underlying bone may have a polished, smooth appearance generally described as eburnation and reflecting subchondral sclerosis. The cartilage that remains is often somewhat discolored and, instead of its usual smooth and shiny appearance, is rough and velvety. When the joint is sectioned, in addition to sclerosis, fibrous-walled cysts filled with mucoid fluid may be seen immediately below the surface. At the periphery of the joint, bony outgrowths, or osteophytes, are frequently present (Fig. 6.11).
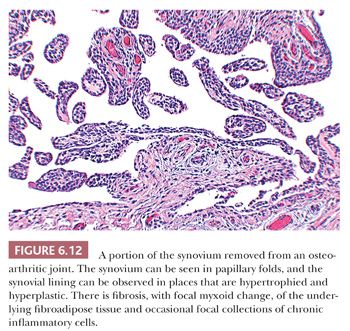
In most cases of osteoarthritis, microscopic evidence of both injury and repair are present in the cartilage and bone without any significant inflammatory component, although occasionally scattered perivascular lymphocytes and even lymphoid follicles may be seen within the synovial membrane (Fig. 6.12) (7).
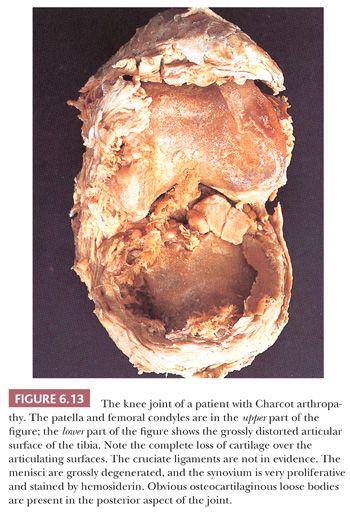
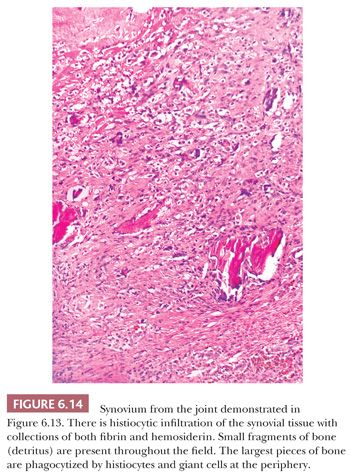
In some neuropathic conditions, such as tabes dorsalis and the peripheral neuropathies of diabetes or pernicious anemia, one may observe rapidly destructive osteoarthritis complicated by multiple loose bodies, severe subluxation, and even dislocation. This type of joint is usually referred to as a Charcot joint (8) and is characterized histologically by extreme deformity and bone and cartilage detritus seen within the synovial membrane, so-called detritic synovitis (Figs. 6.13 and 6.14). Such findings are common in the clinical scenario of “synovial cysts” in the extradural space, often in the lumbar spine, reflecting arthropathy of the associated facet joints.
ARTHRITIS SECONDARY TO SUBCHONDRAL INSUFFICIENCY FRACTURE
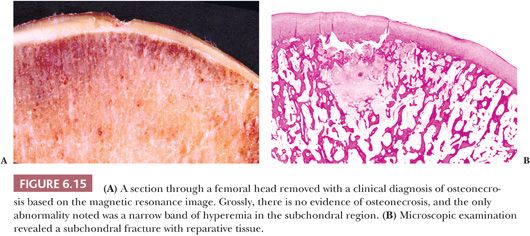
Primary subchondral fractures in the femoral head occur in stress osteopathy in young military trainees; similar fractures termed insufficiency fractures occur in individuals with osteopenia, most commonly in the elderly with osteoporosis. Individuals with osteomalacia and renal transplantation recipients are also at risk. Differentiation of this fracture type from osteonecrosis (avascular necrosis) is possible on magnetic resonance imaging and is of paramount importance, as the initial diagnosis will affect management of the patient. Many clinically reported cases have resolved after conservative therapy without progressing to collapse or requiring surgery. Additionally, as there may be underlying risk factors, these should be sought and treated, as patients may go on to experience contralateral disease or fractures elsewhere. Histopathologically, the most characteristic finding is the presence of fracture callus and granulation tissue along both edges of a fracture line, paralleling the articular surface (Fig. 6.15). There is no evidence of a “V”-shaped area of necrosis of otherwise normal host bone and bone marrow (9,10).
The rapidly destructive arthrosis of the hip joint, first reported in the literature by Postel and Kerboull in 1970 (11), seen mostly in elderly women and characterized by rapid joint destruction within 6 to 12 months with disappearance of the joint space likely represents the end stage of subchondral insufficiency fracture. In these individuals, there is a subtle saddle-shaped deformity of the femoral head with complete loss of the cartilage. Round to oval foci of granulomatous tissue are usually observed in the marrow spaces, in which small fragments of bone and articular cartilage embedded in amorphous eosinophilic debris are found surrounded by aggregated epithelioid histiocytes and giant cells (9,10,12). An accompanying florid detritic synovitis is common. It is likely that in most cases, rapidly destructive arthrosis of the hip and other joints is the result of insufficiency fracture.
OSTEONECROSIS
Osteonecrosis is a common late complication of fractured neck of the femur and, together with idiopathic segmental subchondral infarction, is a significant cause of hip arthritis. In idiopathic disease, not only the hip but also the knee and other major joints may be affected (13). The clinical symptoms result from fractures through the articular surfaces and follow collapse of the underlying necrotic infarcted bone segment. Additionally, there is reparative granulation tissue formation around the margin of the infarct, which destroys the bone at this site and probably contributes to collapse (14–16).
Multiple factors contribute to osteonecrosis, some related to altered fat metabolism and some to microvascular changes. Risk groups include patients who have been treated with corticosteroids, alcoholics, and persons affected by sickle cell disease or Gaucher disease. More than 50% of cases exhibit bilateral or other joint involvement, a feature indicating the systemic nature of the disease.
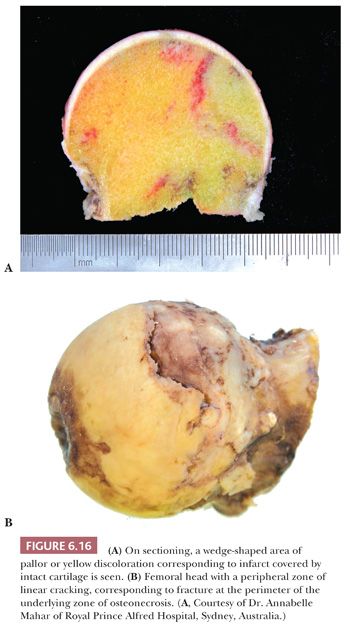
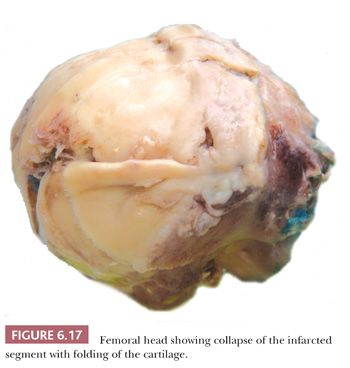
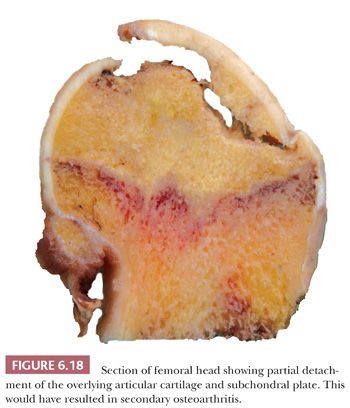
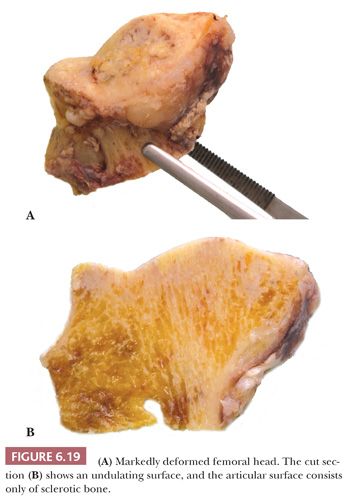
Gross examination of the articular surface of a joint resected from a patient with early clinical osteonecrosis is likely to reveal a fairly intact articular cartilage, although the cartilage is often folded and cracked at the edge of the necrotic area as a result of fracture. This may have a partly circumferential linear arrangement (Fig. 6.16). Section through the infarcted zone shows a characteristic yellow, opaque, chalky appearance in the affected subchondral bone and, at the periphery of the infarct, a rim of hyperemic fibrous tissue is present. Beyond the infarcted zone, the bony trabeculae are frequently thickened by reparative bone formation, giving rise to the increase in density seen on clinical radiographs. Fractures at the junction of the infarct usually result in collapse of the infarcted segment with folding of the cartilage (Fig. 6.17); moreover, with progression of the disease, one sees flattening of the joint surface, detachment of the overlying articular cartilage (to which the subchondral plate remains attached), and loss of bony tissue leading to secondary osteoarthritis (Fig. 6.18). A saddle-shaped deformity is the characteristic macroscopic finding (Fig. 6.19).
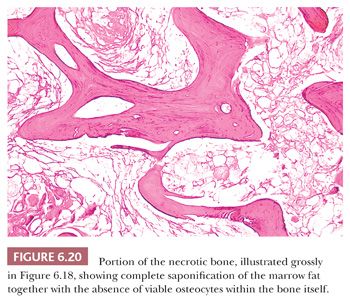
The microscopic recognition of necrotic bone depends mostly on the identification of necrosis of the marrow fat and associated hematopoietic tissue. In the bone itself, the osteocytic lacunae may be enlarged and empty, or they may be of normal size with pyknotic nuclei, depending on the time span over which the necrosis occurred (Fig. 6.20).
Although subchondral osteonecrosis is most often encountered clinically as a primary event leading eventually to severe arthritis, osteonecrosis may also secondarily complicate existing cases of arthritis, in which the necrotic bone will usually show evidence of prior remodeling with sclerosis (17).
Accurate distinction of osteonecrosis from subchondral insufficiency fracture (previously often confused on imaging and histology) is imperative given their differing management and outcomes.
INFLAMMATORY ARTHRITIS
Although the clinical evolution, distribution of disease, and serologic findings differ, the histologic appearances of the many forms of seronegative and seropositive arthritis are similar. New bone formation and sclerosis are common in seronegative arthritis, osteoporosis is a feature of rheumatoid disease in particular.
RHEUMATOID ARTHRITIS
Rheumatoid arthritis is a relatively common inflammatory disease affecting men and women of all ages, with a peak incidence in young adults and menopausal women. There is often a genetic predisposition, and the disease is characterized by immune-driven chronic inflammation. Rheumatoid factors are detectable in the serum in most cases, and recently, identification of anti-citrullinated proteins has proven invaluable in diagnosis (18). Although rheumatoid arthritis is a multiorgan disease, the main target organ is the synovial lining of joints, bursae, and tendon sheaths. It follows a variable course, including exacerbations and remissions of disease activity, many cases are chronic and progressive and result in severe disability and sometimes death.
In the acute stage of rheumatoid arthritis, aspiration of the joint may reveal an inflammatory exudate with many polymorphonuclear leukocytes, even suggesting the possibility of septic arthritis. In general, the white blood cell counts in rheumatoid arthritis are lower (<75,000 per mm3) than those seen in acute septic arthritis (>100,000 per mm3).
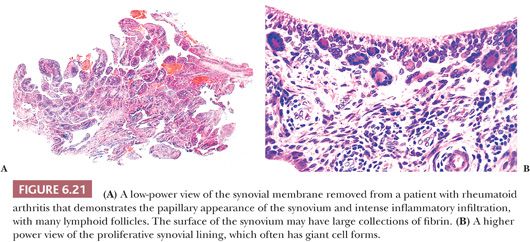
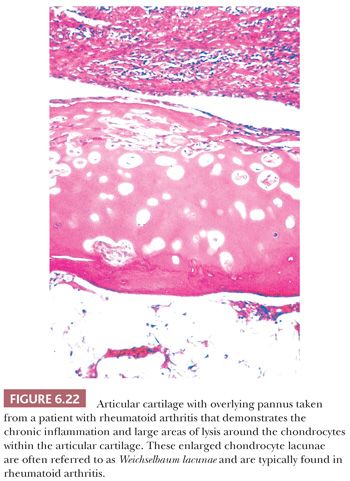
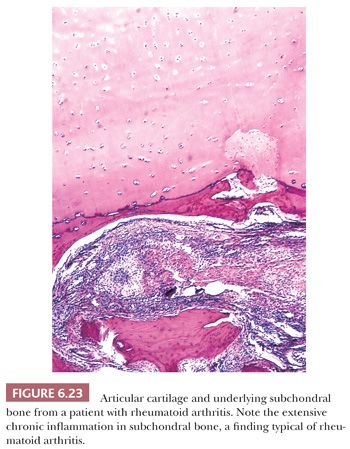
The principal morphologic feature of rheumatoid arthritis is joint destruction, and, unlike osteoarthritis, there is little evidence of reparative tissue, proliferative cartilage, bone sclerosis, or osteophytes (11). The most characteristic microscopic feature is a chronic exudative inflamed synovium characterized by hypertrophy and hyperplasia of the synovial lining cells with focal fibrinous exudation on the surface and an infiltration of the superficial synovium by lymphocytes and plasma cells (Fig. 6.21). Many of the plasma cells contain smooth eosinophilic cytoplasmic inclusions or Russell bodies. Lymphoid follicles are not uncommon. The inflamed synovium eventually extends over the articular surface from the joint periphery to form a covering or pannus that destroys the underlying cartilage both by interfering with chondrocyte nutrition and by enzymatically degrading the matrix, a process known as chondrolysis (Fig. 6.22). The end result of this inflammatory destruction of the articular surfaces may be fusion of the joint either by fibrous granulation tissue or, rarely, by bone (19–21). A chronic inflammatory infiltrate is also typically present in the subchondral bone, and, on occasion, the articular cartilage is destroyed not only from above by the inflamed synovium but also from below by chronically inflamed tissue in the subchondral region (Fig. 6.23).
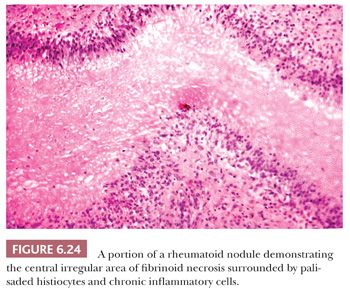
In addition to the destruction of the articular surface by the inflamed synovium, there also is extension of the inflamed synovium into the capsular and periarticular supportive tissues. Destruction of these tissues results in instability of the joint, leading to joint subluxation and the severe deformity so characteristic of rheumatoid arthritis. Approximately 25% of patients with rheumatoid arthritis develop rheumatoid nodules. These nodules are found in subcutaneous sites, especially along the extensor surfaces of the forearm and the elbow, as well as the shin. Less often, they may occur in visceral organs, especially the heart, lung, and gastrointestinal tract, and even in the synovial membrane itself. Histologically, the rheumatoid nodule is characterized by an irregular central zone of fibrinoid necrosis surrounded by palisaded histiocytes, giant cells, and a cuff of chronic inflammatory cells (Fig. 6.24). On polarization, the collagenous nature of the central region of the nodules can be discerned.
With the destruction of the articular cartilage, the inflammatory component tends to regress, and in patients who have had total joint surface replacements, even without synovectomy, the synovitis in that joint tends to become much less severe. Conversely, in a rheumatoid joint that has been treated by synovectomy without resection of the articular bone, the inflammatory synovium may reappear; the progression of the disease is only temporarily halted by synovial excision.
Involvement of the spine occurs in more than 50% of patients with rheumatoid arthritis. Most commonly, the cervical spine is involved. Bony erosion and apophyseal joint space narrowing are common and may result in ankylosis. Erosions of the odontoid process occur in approximately one-third of patients as a result of weakening of the transverse and apical ligaments by the inflamed synovium. Therefore, minor trauma may result in atlantoaxial subluxation or basilar invagination and sudden death.
Although synovitis in seronegative forms of inflammatory arthritis is similar to that in rheumatoid disease, neutrophils may form part of the infiltrate, and osteosclerosis of the subchondral bone is more commonly observed. However, in general, distinction between seronegative and seropositive types of true inflammatory arthropathy is not possible on histopathologic grounds alone.
In all cases of synovitis, the possibility of crystal deposition as a cause should be considered. The main forms of crystal-induced synovitis are gout, calcium hydroxyapatite deposition disease, and calcium pyrophosphate deposition disease, which are discussed below.
GOUT
Clinical gout is the result of an inflammatory response to monosodium urate monohydrate crystals that may form as a result of hyperuricemia. Gout usually occurs in middle-aged to elderly adults, with a current incidence of 1% to 2% in adults in Western countries; gout is on the rise in many countries (22,23). Prevalence rates have been reported as much higher in Asian populations and among Aboriginal Australians and Maoris, being present in 7% and 8.8% of adult males from the latter two groups, respectively (24,25). Hyperuricemia, which forms part of the metabolic syndrome, may result from diet, increased cell breakdown associated with malignancy, or genetic factors. Contributing factors are hypertension, renal impairment, and the use of diuretics.
In both the primary and the secondary forms of gout, hyperuricemia results in the deposition of monosodium urate crystals in joints and visceral organs (26,27). The kidney is frequently affected, and uric acid stones are present in nearly all patients with gout.
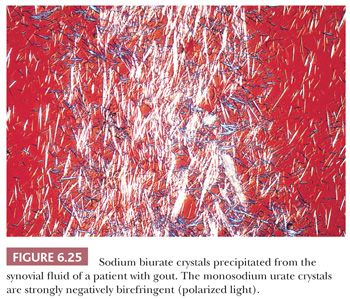
Acute gouty arthritis is generally monoarticular and has a predilection for the lower extremity, particularly the first metatarsophalangeal joint. In the early stages of the disease, the patient’s symptoms may be attributed to sepsis. However, examination of the aspirated fluid by polarized light microscopy will, in all cases, demonstrate the presence of strongly negatively birefringent needle-shaped crystals (Fig. 6.25) (28).
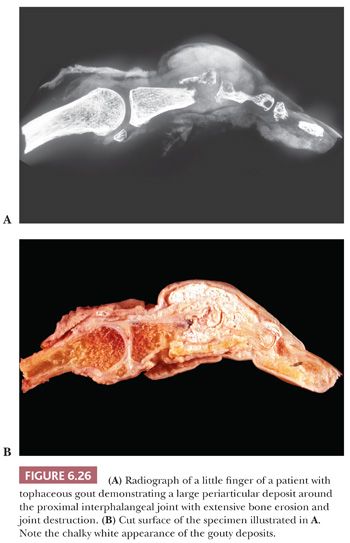
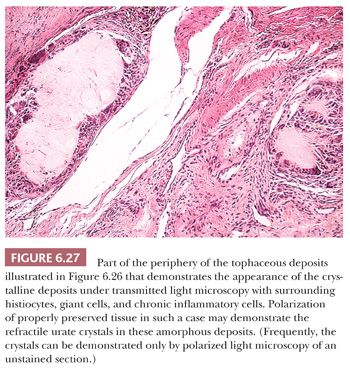
Continued deposition of monosodium urate crystals eventually results in chronic tophaceous gout. Tophaceous (chalky) deposits around joints ultimately erode into the articular cartilage and bone and cause large periarticular defects that may be seen on radiologic examination (Fig. 6.26) and on occasion can even be mistaken for an aggressive neoplasm. Histologically, the resected tophi consist of large crystalline deposits rimmed by histiocytes and giant cells and surrounded by fibrous tissue (Fig. 6.27). During the course of routine processing in formalin and subsequent H&E staining of histologic sections, the crystals, which are water soluble, are usually removed; however, examination of either unstained sections or sections stained with eosin-only by polarized light generally demonstrates the crystalline nature of the deposits. If crystal deposition disease is suspected clinically, we recommend the material be sent fresh. We then fix and process it in absolute alcohol. Direct microscopic evaluation of synovial fluid remains the mainstay of diagnosis, and aspiration of joint fluid to obtain such a sample may be conclusive (Table 6.1).

CALCIUM PYROPHOSPHATE CRYSTAL DEPOSITION DISEASE
Small deposits of a chalky white material within the articular tissues are a common finding in arthritic joints and are frequently the result of calcium pyrophosphate deposition disease (CPPD). The disease may be familial in patients with ANKH mutations or may be associated with other metabolic diseases where inhibition of pyrophosphatase by divalent ions occurs (e.g., hypercalcemia in hyperparathyroidism, iron in hemochromatosis, copper in Wilson disease) or even gout. However, most cases lack such associations. In community studies, the prevalence rates are 8.1% in the United States and 7.0% in the United Kingdom (29,30). CPPD is virtually endemic in regions of Slovakia, Chile, and the Netherlands (31,32).
The nomenclature of this disease has been confusing. The term chondrocalcinosis has been used by radiologists to describe calcium deposits in the knee menisci of elderly patients. The term pseudogout has been used by rheumatologists to describe those patients with CPPD who present with an acutely swollen joint mimicking gout. Very rarely, a patient with CPPD will present with a tumorlike mass (tumoral CPPD or tophaceous pseudogout) (33). The most common clinical presentation of a patient with CPPD is similar to that of a patient with osteoarthritis; approximately 50% of affected patients present with progressive arthritis that often affects several joints. The onset of the disease is usually in the fourth decade of life (34–38).
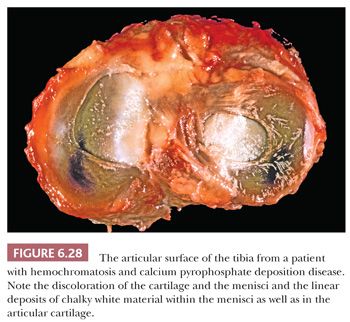
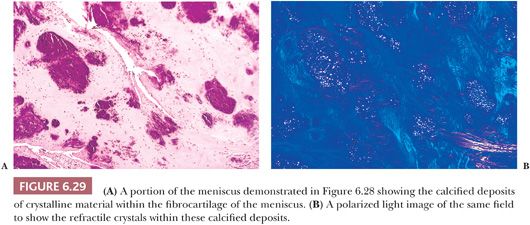
Gross examination of the affected tissues reveals small chalky white deposits (Fig. 6.28), which on microscopic examination, in contrast to uric acid deposits, have a purple brushlike quality on H&E. They are commonly seen in and indeed evolve in a background of chondroid metaplasia. They are composed of small rhomboidal crystals that are weakly positively birefringent. Like uric acid crystals, they may be diminished with processing and can be retained and accentuated with eosin-only stain (Fig. 6.29). Because on occasion there may be some histiocytic and giant cell reaction around these crystalline deposits, it is important that they be distinguished from uric acid crystals (Table 6.1).
HYDROXYAPATITE DEPOSITION AND RELATED CONDITIONS
Deposition of calcium hydroxyapatite in soft tissues, which must be distinguished from gout and CPPD, may be associated with trauma, scleroderma, renal failure, hyperparathyroidism, sarcoidosis, metastatic disease, myeloma, or hypermetabolic states (39–41).
Metastatic calcification is a problem particularly in patients with disturbed calcium homeostasis and hypermetabolic states (e.g., hyperparathyroidism and Paget disease), who may have undergone prolonged bed rest. The calcification, which may be both intracellular and extracellular, is likely to occur in the kidneys, alveolar lining of the lungs, cornea, conjunctiva, and gastric mucosa (i.e., those areas subject to large pH changes) as well as in the media and intima of blood vessels.
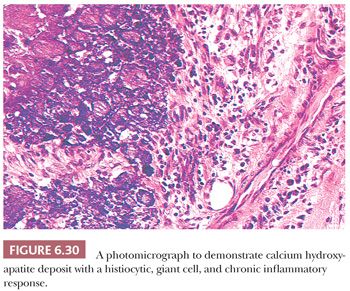
In foci of coagulation necrosis, caseous necrosis, and fat necrosis, the dead tissue that does not undergo rapid absorption frequently becomes calcified. This type of calcification, which is not related to any disturbance in calcium homeostasis, is called dystrophic calcification. Of particular interest to orthopedic surgeons is the calcification that is common in tendons, ligaments, and bursae, presumably after mechanical trauma. A common clinical presentation is a painful shoulder that corresponds anatomically to the insertion of the supraspinatus muscle onto the humerus. Gross examination reveals amorphous chalky white deposits, and microscopic studies reveal that the calcium is isolated in fibrous or fatty tissue or may be present in association with chronic inflammatory cells including, at times, multinucleated giant cells (Fig. 6.30). Characteristically, background features of tendinosis can be seen (see the following discussion).
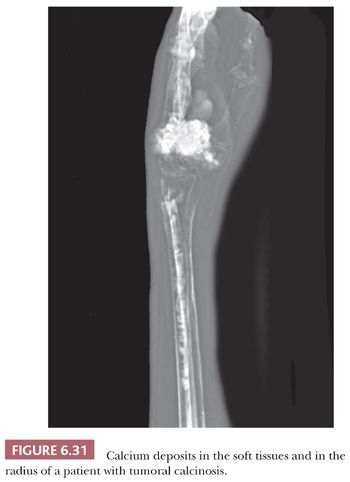
Tumoral calcinosis is a rare condition that mostly affects blacks in otherwise good health. Typically, blood phosphate levels are elevated. The disease usually presents in the second decade of life and is characterized by deposition of painless calcific masses around the hips, elbows, shoulders, and gluteal area (i.e., areas subject to movement or pressure). Rarely, intraosseous deposits also may occur (Fig. 6.31) (42). Familial cases are associated with germline mutations in GALNT3 and, more rarely, FGF23 or KL (KLOTHO) (43–45), resulting in impaired renal phosphate excretion.
SEPTIC ARTHRITIS
Joint infection may be caused by hematogenous infection of the synovium, decompression of contiguous osteomyelitis, or direct inoculation of a joint after trauma or surgery. With total joint replacement and arthroscopy now such common procedures, postsurgical infection is one of the most common causes of the septic joint.
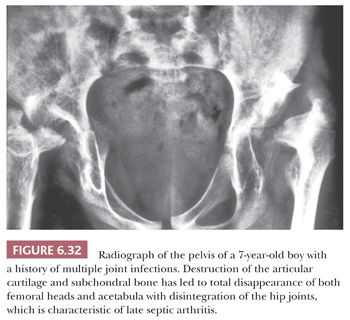
Septic arthritis is relatively common in neonates and infants, in whom it affects the hip, knee, or ankle, and severe residual growth disturbances may result from damage to the growth plate (Fig. 6.32) (46,47). Other groups of patients particularly susceptible to septic arthritis are debilitated older adults with rheumatoid arthritis or other chronic inflammatory joint diseases and immunocompromised patients. The diagnosis is established by joint aspiration. To increase the likelihood of bacterial growth, the aspirate should be inoculated into the culture medium as soon as possible after collection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


