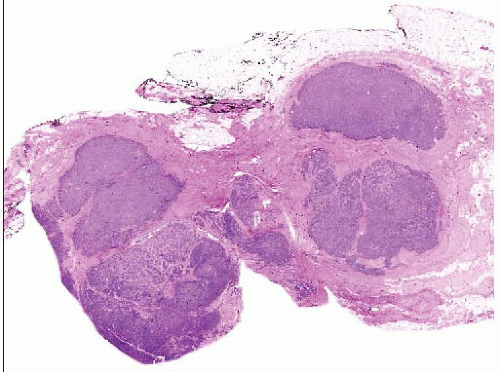
Invasive Lobular Carcinoma Variants
Key Facts
Terminology
Variant forms differ from classical ILC with regard to architecture &/or cytology
Show areas of nonlinear infiltration
Variable degree of atypia and high-grade nuclei
All lack cohesion either due to loss of E-cadherin or other cell adhesion molecules
Clinical Issues
Classical ILC has better prognosis than variant forms of ILC
Grade may be better method of subclassifying ILC and is related to variant type
Majority of classical ILC is moderately or well differentiated
Solid ILC is predominantly poorly differentiated and alveolar ILC moderately differentiated
Microscopic Pathology
Variant forms of ILC can be classified based on differences in architectural growth pattern and cytologic features
Architectural variants
Classical (55%)
Solid (5-10%)
Alveolar (5-20%)
Cytologic variants
Pleomorphic
Histiocytoid
Top Differential Diagnoses
Invasive ductal carcinoma
Lymphoid neoplasms
Metastatic melanoma
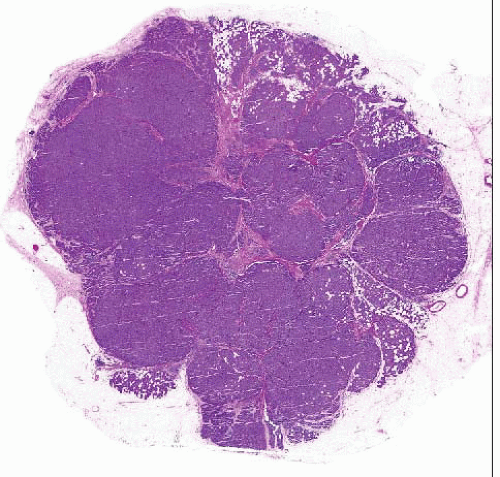 The majority of invasive lobular carcinomas invade as files of single cells. However, cytologically identical cells can grow in other patterns, such as this solid type of invasive lobular carcinoma. |
TERMINOLOGY
Abbreviations
Variant forms of invasive lobular carcinoma (ILC)
Histologic variants of ILC
Classical variant (ILCCV)
Solid variant (ILCSV)
Alveolar variant (ILCAV)
Cytologic variants of ILC
Pleomorphic variant (ILCPV)
Histiocytoid variant (ILCHV)
Definitions
Variant forms differ from classical ILC with regard to architecture &/or cytology
May show substantial elements of nonlinear infiltration and growth
May show significant atypia with high-grade nuclei
Focal areas of classical ILC with linear growth pattern can be found in most variant forms
Predominant pattern (> 80%) determines histologic type of ILC
ETIOLOGY/PATHOGENESIS
Molecular Pathology
Most ILCs, including variant types, show complete E-cadherin inactivation
Similar mechanisms of E-cadherin inactivation have been described in classical and variant forms of ILC
E-cadherin gene (CDH1) has been reported to be frequently mutated in all variants of ILC
Remaining wild-type CDH1 allele is inactivated by loss of heterozygosity (LOH) or promoter hypermethylation at CDH1 locus (16q22.1)
In approximately 10% of ILC, E-cadherin is expressed but cells are discohesive
Other components of cell adhesion, such as catenins, may be nonfunctioning
Majority of ILC are ER/PR positive and in luminal molecular subgroup by gene profiling
ILCPV may display luminal, molecular apocrine, or HER2 subgroups by gene profiling
CLINICAL ISSUES
Natural History
ILC and variant forms account for 5-15% of invasive breast cancers
Outcome of patients with ILC does not appear to be significantly different from that of patients with carcinomas of no special type
Similar prognosis when these histologic types of breast cancer are matched by stage and grade
ILC shows proclivity for metastatic dissemination to specific anatomic sites
Gastrointestinal tract, uterus, meninges, ovary, serosal cavities, and bone
Less frequent metastasis to lung and pleura
ILCPV shows increased tendency for local recurrence after conservative treatment compared with ILCCV
Treatment
Treatment of all ILC and variant forms is dependent on tumor stage and parallel to treatment for IDC
Endocrine and HER2-targeted therapies are dependent on results of biomarker studies for these factors
Most (but not all) ILC will be ER positive
Overexpression of HER2 rare (< 1%) in ILCCV
48-80% of ILCPV may be HER2 positive
In neoadjuvant studies, ILC is less likely than nonlobular carcinomas to show pathologic complete response to chemotherapy
Prognosis
Most studies suggest that classical ILC has better prognosis than variant forms of ILC
Differences have not been statistically significant in many reports
No reproducible differences among patients with nonpleomorphic variants
Classical ILC appears to have much more favorable prognosis than pleomorphic variant
ILCPV is reported to have more aggressive tumor biology and clinical behavior compared with other types
Histologic grading of ILC (Nottingham grading system) is recommended for all tumors
In retrospective series, histologic grade provides strong predictor of outcome
MICROSCOPIC PATHOLOGY
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree