Chapter 38 Introduction to Endocrine Pharmacology and Hormones of the Hypothalamus and Pituitary Gland
| Abbreviations | |
|---|---|
| ACTH | Adrenocorticotropic hormone |
| AVP | Arginine vasopressin, antidiuretic hormone |
| cAMP | Cyclic adenosine monophosphate |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| DHEA | Dehydroepiandrosterone |
| DHT | Dihydrotestosterone |
| DI | Diabetes insipidus |
| DNA | Deoxyribonucleic acid |
| Epi | Epinephrine |
| FDA | United States Food and Drug Administration |
| FSH | Follicle-stimulating hormone |
| GH | Growth hormone |
| GHRH | Growth hormone-releasing hormone |
| GI | Gastrointestinal |
| GnRH | Hypothalamic gonadotropin-releasing hormone |
| hCG | Human chorionic gonadotropin |
| hGH | Human growth hormone |
| hMG | Human menopausal gonadotropin |
| IGF-1 | Insulin-like growth factor-1 |
| IM | Intramuscular |
| IV | Intravenous |
| LH | Luteinizing hormone |
| RNA | Ribonucleic acid |
| SC | Subcutaneous |
| SRIF | Somatostatin, somototropin-release inhibiting hormone |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
The endocrine system is a complex communication system responsible for maintaining homeostasis throughout the body, and it is vital to individual and species survival and propagation as well as adaptation to the environment. The system consists of a diverse group of ductless glands that secrete chemical messengers called hormones into the circulation. The secreted hormones are transported in the bloodstream to target organs, where they act to regulate cellular activities. For a hormone to elicit a response, it must interact with specific receptors on the cells of the target organ, much like the interaction between neurotransmitters and receptors involved in the process of neurotransmission in the central and peripheral nervous systems (see Chapters 9 and 27). Receptors play a key role in the mechanisms of action of endocrine hormone systems; key receptor mechanisms pertinent to endocrine systems are summarized in Chapter 1.
Hormones are chemically and structurally diverse compounds and can be divided into three main classes based on chemical composition, viz., the amino acid analogs, the peptides, and the steroids. The amino acid analogs, often termed amine hormones, are all derived from tyrosine and include epinephrine (Epi) and the iodothyronines or thyroid hormones. The peptide hormones are subclassified on the basis of size and glycosylation state and may be single- or double-chain peptides. The steroid hormones are all derived from cholesterol and may be subclassified as adrenal steroids or sex steroids, the former synthesized primarily in the adrenal cortex and the latter synthesized in the ovaries or testes. The major endocrine glands and their associated hormones are listed in Box 38-1.
Hormones are generally distinguished from other types of modulatory factors (i.e., neurotransmitters) by a longer duration of effect and more extensive circulation in the body. While in the circulation, a hormone is frequently associated with one or more types of transport proteins from which it must dissociate to interact with responsive receptors. In addition, availability to tissues is dependent upon membrane exclusion mechanisms, susceptibility to tissue modification, and ultimately the rate of renal or hepatic metabolism, inactivation, and excretion. As mentioned, hormones exert their effects by binding to and activating receptors on target cells. These receptors can be located on the cell surface, as for peptide hormones, or within the cell, as in the case of steroids and thyroid hormones. After receptor activation, intracellular signaling pathways (e.g., second messenger systems or ligand-activated transcription factors) are modulated, which acutely or chronically alter cellular physiology and potentially whole organism physiology.
Excessive levels of endogenous hormones may result from excessive organ secretion or unregulated ectopic formation. Hormone overproduction by a secreting organ is commonly associated with excessive stimulation or a malignancy (or hyperplasia). Successful management of this situation includes blockade of the stimulatory agent, if identifiable, or interference with hormone formation, secretion, or action. Ectopic production of a biologically active form of the hormone by tissues is complicated by the lack of feedback mechanisms to regulate hormone production and is typically associated with tissue malignancy or infection. The primary determinant of successful intervention frequently requires a combination of ablation of the secreting tissue and pharmacological agents to antagonize the effects of elevated hormone levels. The success of this technique hinges on the ability of the responsible tissue to respond to pharmacological intervention. If it is not possible or detrimental to directly reduce hormone levels, a situation often encountered before or immediately after surgery, or when the cause of the elevated hormone levels is unknown or uncorrectable, alternative, patient-specific strategies to reduce the effects of elevated hormone levels must be used. A summary of strategies to manage the levels and action of hormones is presented in Box 38-2. A list of drugs that affect hormonal balance and their mechanisms of action are in Box 38-3.
BOX 38–2 Strategies to Manage the Levels and Action of Hormones
BOX 38–3 Drugs Known to Affect Hormonal Balance
Effectors of Hormone Release/Reuptake
Steroid Biochemistry and Physiology
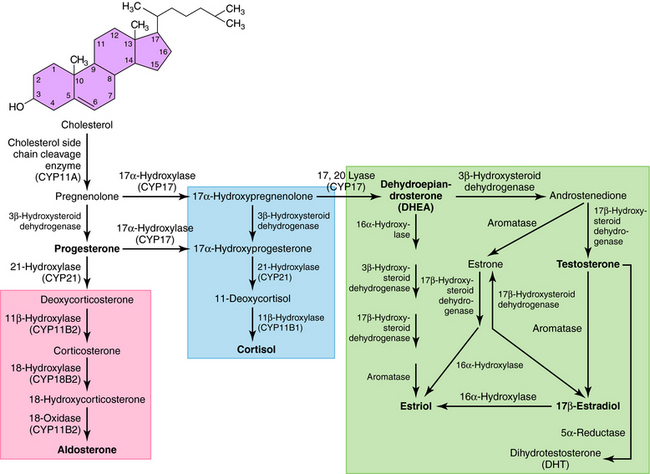
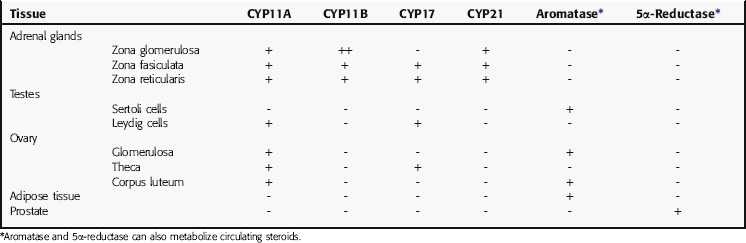
All secreted steroids are synthesized from cholesterol, which can be synthesized de novo or derived from circulating lipoproteins. Similar metabolic pathways mediate steroid synthesis in all organs (Fig. 38-1). The organ-specific formation of secreted steroids depends on the presence of specific catalytic enzymes (Table 38-1).
TABLE 38–1 Enzymes Present in Different Tissues Mediating the Organ- or Tissue-Specific Formation of Steroid Hormones
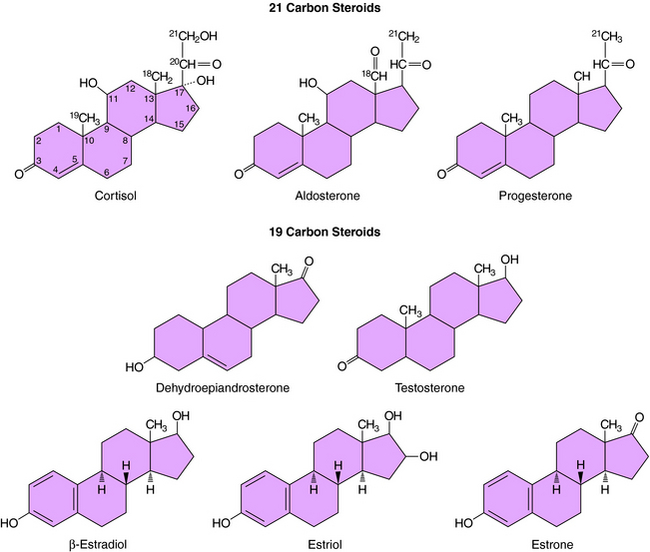
The action of steroids is mediated largely by altering gene transcription through interaction with promoter deoxyribonucleic acid (DNA) of genes. Steroid receptors are dimeric and coupled with accessory proteins until activated by ligands outside the nucleus. The steroid-receptor complex is phosphorylated and translocated to the nucleus through a nuclear pore, facilitated by the importin protein. The interaction with the gene promoter region occurs through steroid-specific palindromic nucleotide sequences within the receptor. The interaction of DNA and the steroid-receptor complex is dependent on steroid structural differences, amino acid sequence of the DNA binding domain, the nucleotide sequence of the DNA binding site, and the architecture of the gene promoter. The structures of the primary circulating steroids are shown in Figure 38-2.
In the adrenal gland, the primary secreted steroids are aldosterone, cortisol, and dehydroepiandrosterone (DHEA) (see Fig. 38-2). Aldosterone is the primary mineralocorticoid and acts at the luminal epithelia to promote renal reuptake of Na+, which conserves Na+ and can elevate blood pressure. In the zona glomerulosa, the lack of CYP17 is associated with nearly exclusive formation of aldosterone. Further, the release of aldosterone from the zona glomerulosa is regulated by the renin-angiotensin pathway as a result of activation angiotensin II-receptors, which are linked to the formation of 1,4,5-inositol triphosphate. The amount of aldosterone released is relatively low (50 to 150 µg/day); aldosterone is transported in the blood through an interaction with albumin with a bound/free ratio of 70/30.
The complement of enzymes in the zona fasciculata and zona reticularis permits the formation of cortisol, the primary circulating glucocorticoid (see Chapter 39). The release of cortisol is dependent on a tightly regulated hypothalamic-anterior pituitary-adrenal cortex axis. The biological role of glucocorticoids is complex and temporal. The liver has the greatest level of nuclear receptors or steroid-activated transcription factors, although they are present in many tissues. The primary systems affected by cortisol include self-regulation of formation via suppression of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secretion, storage of hepatic glycogen, response to stress, and suppression of the immune system. The daily production of cortisol ranges from 10 to 20 mg, and plasma levels follow a diurnal pattern with the highest levels in the morning. In the blood, cortisol is bound to a specific hepatic protein, corticosteroid-binding protein (aka transcortin), which promotes its transport and increases its duration of action.
The secretion of estrogen (β-estradiol) and progesterone from the ovary is regulated by hypothalamic gonadotropin-releasing hormone (GnRH), anterior pituitary follicle-stimulating hormone (FSH), and leutenizing hormone (LH). Release of the gonadotropic hormones and the ovarian steroids during the menstrual cycle is episodic, and the highest levels of β-estradiol or progesterone occur during the late follicular phase or midleutal phase, respectively (see Chapter 40). Most of these circulating steroids (98%) are bound to specific steroid hormone-binding globins.
The primary testicular androgen, testosterone, is converted to dihydrotestosterone (DHT) in tissues expressing 5α-reductase. The actions of androgens include development of male reproductive tract and accessory tissues, stimulation of secondary sexual traits, growth, and development of the central nervous system (CNS) (see Chapter 41). As shown in Figure 38-1, the expression of steroid metabolizing enzymes promotes the formation of DHEA and androstenedione leading to the formation of testosterone; the expression of aromatase in ovarian cells permits conversion of testosterone to β-estradiol.
Therapeutic Overview
Pharmacology of Hypothalamic and Pituitary Hormones
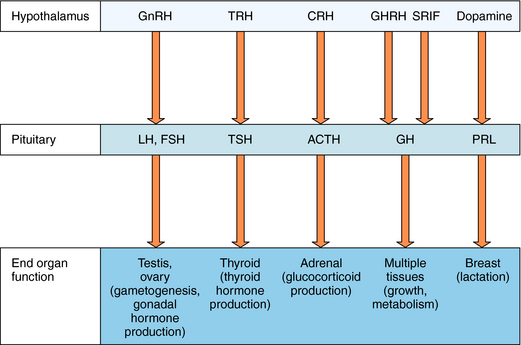
The hypothalamus and pituitary gland work in concert to regulate endocrine systems throughout the body. Peptides and biogenic amines synthesized and secreted by specialized neurons within the hypothalamus are transported to the anterior pituitary by the hypothalamic-hypophyseal portal circulation, where they act through specific receptors to stimulate or inhibit hormone secretion (Fig. 38-3). Anterior pituitary hormones trigger peripheral endocrine organs to produce hormones, which have individual functions and provide feedback to the hypothalamus and pituitary to regulate the synthesis and release of their tropic hormones. As mentioned, GnRH (also called luteinizing hormone releasing hormone) stimulates the secretion of LH and FSH by the pituitary. LH and FSH promote gametogenesis and gonadal hormone production by the ovaries and testes (see Chapters 40 and Chapter 41). Thyrotropin-releasing hormone (TRH) stimulates secretion of thyroid-stimulating hormone (TSH), which in turn controls thyroid function (see Chapter 42) CRH stimulates the secretion of ACTH, which promotes the secretion of cortisol by the adrenal cortex (see Chapter 39). Growth hormone-releasing hormone (GHRH) stimulates and somatostatin (also called somatotropin-release inhibiting factor, SRIF) inhibits the production of growth hormone (GH), which has numerous effects on growth and metabolism. Hypothalamic dopamine functions to tonically inhibit secretion of prolactin, the hormone primarily responsible for lactation and suppression of fertility while nursing.