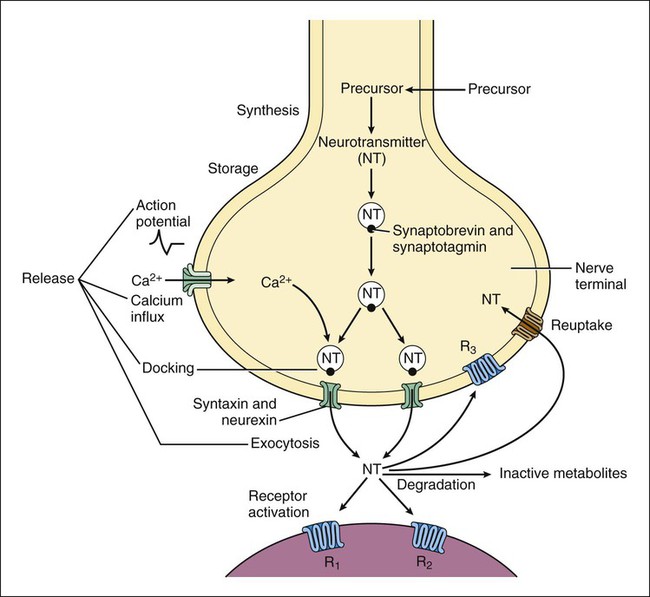
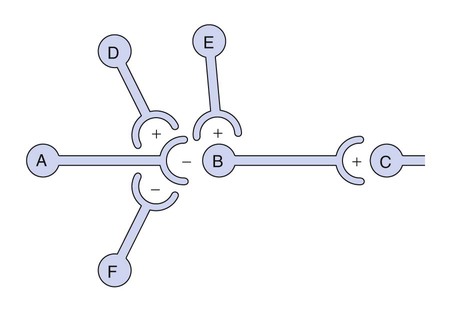
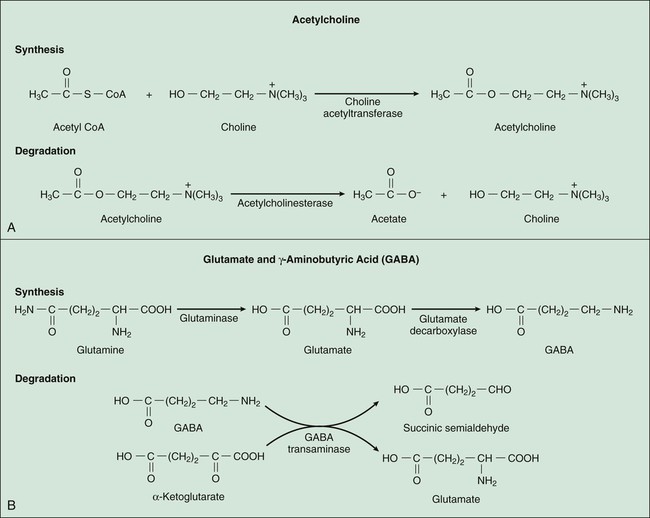
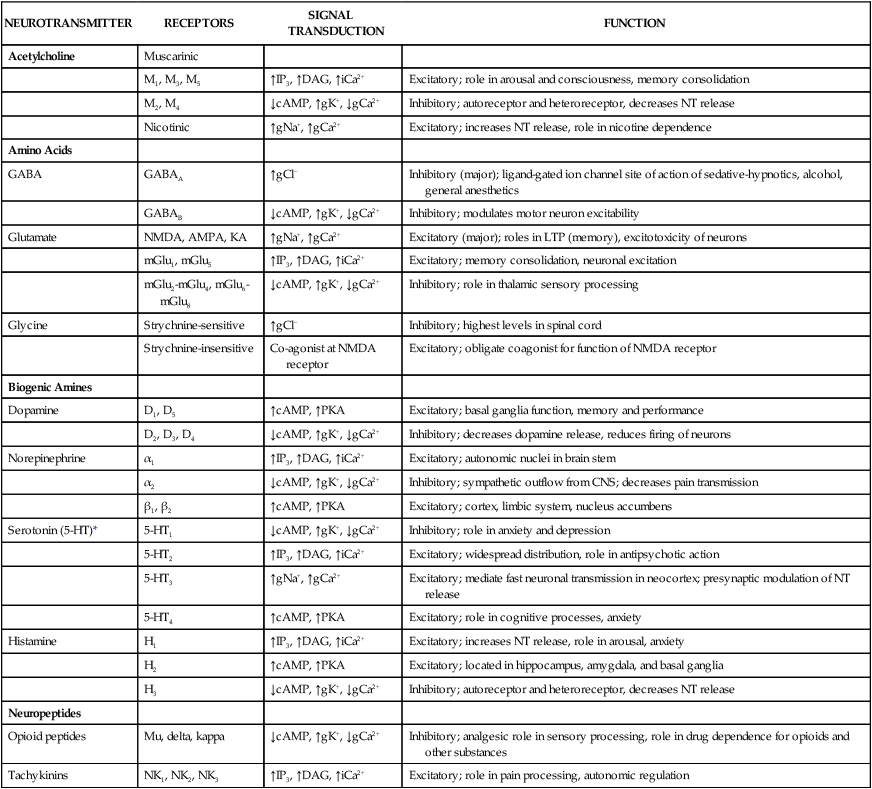
Neurotransmitters are synthesized in neuronal cell bodies or terminals, and they are stored in neuronal vesicles until they are released into a synapse (Fig. 18-1). The release of neurotransmitters is activated by membrane depolarization and calcium influx into the cell. Calcium evokes the interaction of storage vesicle proteins (synaptobrevin and synaptotagmin) and membrane-docking proteins (syntaxin and neurexin) and leads to vesicle fusion with the membrane and exocytosis of the neurotransmitter. As shown in Box 18-1, the interaction of multiple neurotransmitters at a particular site in a neuronal tract enables the complex interplay of various neuronal systems and contributes to the wide range of functional expression exhibited in the CNS. For example, inhibition of the release of an inhibitory neurotransmitter will actually increase neurotransmission in the target neuron. Similarly, drugs can act in complex ways to affect neurotransmission. Ethanol (ethyl alcohol), for instance, can diminish the inhibitory influence of the cerebral cortex on certain human behaviors and thereby increase drug-induced behaviors, a phenomenon called behavioral disinhibition. Ethanol and other CNS depressants, initially or at low doses, exert their effects on the smaller and more numerous inhibitory neurons, creating disinhibition via excitation owing to removal of inhibitory neurotransmitters. At higher doses, larger excitatory neurons are inhibited and profound depression of CNS activity can occur. Neurotransmitters in the CNS can be characterized as slow or fast, depending on the receptors that are activated and the persistence of signal transduction pathways. The best examples of fast neurotransmitters are γ-aminobutyric acid (GABA) and glutamate acting at ligand-gated ion channels. Binding of these amino acid neurotransmitters directly to subunits of the ion channel protein directly initiates ion flow with a signal that lasts for only a few milliseconds. Examples of slow neurotransmitters are norepinephrine and serotonin acting at G protein–coupled receptors. These activated G protein–coupled receptor proteins initiate a slower, multistep process with alterations in second messengers and membrane effects that can last from many milliseconds to as long as a second. A slow (long-acting) signal can influence the overall tone of a neuron because it can modulate the signals of several other fast neurotransmitters acting on the same neuron. For this reason, slow neurotransmitters can also be called neuromodulators. Important neurotransmitters in the CNS include acetylcholine and several amino acids, biogenic amines, and neuropeptides. Table 18-1 lists the names, receptors, mechanisms of signal transduction, and functions of the major neurotransmitters. TABLE 18-1 Major Neurotransmitters and Their Receptors in the Central Nervous System *Over a dozen types of serotonin receptors are cloned; the four given here are the main types. The receptors can be divided into two basic groups: ionotropic receptors, also called ligand-gated ion channels, which are directly associated with ion channels, and metabotropic receptors, which are typical G protein–coupled receptors (see Chapter 3). Although this terminology is most frequently applied to receptors for amino acid neurotransmitters (e.g., GABA and glutamate), it is equally appropriate for other classes of neurotransmitter receptors. The mechanisms of signal transduction for neurotransmitters in the CNS are similar to those for neurotransmitters in the autonomic nervous system. The activation of ionotropic receptors alters chloride, sodium, potassium, or calcium influx and thereby evokes excitatory or inhibitory membrane potentials. The linkage of metabotropic receptors with G proteins leads to activation or inhibition of adenylyl cyclase and alteration in the levels of intracellular cAMP, or activation of phospholipase C and the formation of inositol triphosphate and diacylglycerol. Metabotropic receptor activity can also modulate ion channel activity via second messengers (most notably calcium) that activate protein kinases responsible for the phosphorylation of ion channels. Signal transduction for other receptors in the CNS is discussed in Chapter 3. Acetylcholine, synthesized from acetyl coenzyme A and choline, is degraded to acetate and choline by the enzyme acetylcholinesterase (Fig. 18-2). Acetylcholine receptors (also known as cholinergic receptors) consist of two main types: muscarinic receptors and nicotinic receptors. The properties and mechanisms of these receptors are compared in Table 6-1.
Introduction to Central Nervous System Pharmacology
Neurotransmission in the Central Nervous System
Principles of Neurotransmission
Neurotransmitter Synthesis and Metabolism
Excitatory and Inhibitory Neurotransmission
Fast versus Slow Signals
Neurotransmitters and Receptors
NEUROTRANSMITTER
RECEPTORS
SIGNAL TRANSDUCTION
FUNCTION
Acetylcholine
Muscarinic
M1, M3, M5
↑IP3, ↑DAG, ↑iCa2+
Excitatory; role in arousal and consciousness, memory consolidation
M2, M4
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; autoreceptor and heteroreceptor, decreases NT release
Nicotinic
↑gNa+, ↑gCa2+
Excitatory; increases NT release, role in nicotine dependence
Amino Acids
GABA
GABAA
↑gCl−
Inhibitory (major); ligand-gated ion channel site of action of sedative-hypnotics, alcohol, general anesthetics
GABAB
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; modulates motor neuron excitability
Glutamate
NMDA, AMPA, KA
↑gNa+, ↑gCa2+
Excitatory (major); roles in LTP (memory), excitotoxicity of neurons
mGlu1, mGlu5
↑IP3, ↑DAG, ↑iCa2+
Excitatory; memory consolidation, neuronal excitation
mGlu2-mGlu4, mGlu6-mGlu8
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; role in thalamic sensory processing
Glycine
Strychnine-sensitive
↑gCl−
Inhibitory; highest levels in spinal cord
Strychnine-insensitive
Co-agonist at NMDA receptor
Excitatory; obligate coagonist for function of NMDA receptor
Biogenic Amines
Dopamine
D1, D5
↑cAMP, ↑PKA
Excitatory; basal ganglia function, memory and performance
D2, D3, D4
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; decreases dopamine release, reduces firing of neurons
Norepinephrine
α1
↑IP3, ↑DAG, ↑iCa2+
Excitatory; autonomic nuclei in brain stem
α2
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; sympathetic outflow from CNS; decreases pain transmission
β1, β2
↑cAMP, ↑PKA
Excitatory; cortex, limbic system, nucleus accumbens
Serotonin (5-HT)*
5-HT1
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; role in anxiety and depression
5-HT2
↑IP3, ↑DAG, ↑iCa2+
Excitatory; widespread distribution, role in antipsychotic action
5-HT3
↑gNa+, ↑gCa2+
Excitatory; mediate fast neuronal transmission in neocortex; presynaptic modulation of NT release
5-HT4
↑cAMP, ↑PKA
Excitatory; role in cognitive processes, anxiety
Histamine
H1
↑IP3, ↑DAG, ↑iCa2+
Excitatory; increases NT release, role in arousal, anxiety
H2
↑cAMP, ↑PKA
Excitatory; located in hippocampus, amygdala, and basal ganglia
H3
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; autoreceptor and heteroreceptor, decreases NT release
Neuropeptides
Opioid peptides
Mu, delta, kappa
↓cAMP, ↑gK+, ↓gCa2+
Inhibitory; analgesic role in sensory processing, role in drug dependence for opioids and other substances
Tachykinins
NK1, NK2, NK3
↑IP3, ↑DAG, ↑iCa2+
Excitatory; role in pain processing, autonomic regulation
Acetylcholine
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Introduction to Central Nervous System Pharmacology
Only gold members can continue reading. Log In or Register to continue