INTRODUCTION
In Chapters 20–25, cardiovascular pharmacology is considered in the context of individual physiologic systems. For example, diuretics are discussed in the context of volume regulation, while inhibitors of angiotensin converting enzyme (ACE) are discussed in the context of vascular tone. However, the clinical presentation of cardiovascular diseases often involves interactions among these individual systems. As a result, pharmacologic management often necessitates the use of agents from several drug classes. This chapter presents three common cardiovascular disease states—hypertension, ischemic heart disease, and heart failure—in a single, longitudinal clinical case. For each disease, an understanding of the disease pathophysiology underscores the rationale for pharmacologic interventions and may also highlight the potential for adverse effects (such as serious drug–drug interactions). This chapter aims to integrate pathophysiology with pharmacology to provide a thorough and mechanistic understanding of the contemporary management of these common cardiovascular disease states.

Thomas N, a 45-year-old manager at a telecommunications company, presents to the cardiology clinic for evaluation of exertional shortness of breath. Mr. N had always been zealous in maintaining aerobic fitness, but about 6 months before his cardiology clinic visit, he began to note severe breathlessness as he approached the completion of his daily run, which concludes with a long but gentle uphill climb. During the intervening 6 months, the patient reports a progression in his symptoms to the point that, now, he rarely completes the first half of his daily run without resting. He denies chest discomfort at rest or with exercise. His family history is notable for hypertension and premature atherosclerosis. Mr. N has never used tobacco products.
On examination, the patient is hypertensive (blood pressure, 160/102 mm Hg), and a prominent presystolic S4 is heard at the left ventricular apex. The exam is otherwise unremarkable. The chest x-ray is reported as normal. The electrocardiogram (ECG) reveals normal sinus rhythm with voltage criteria for left ventricular hypertrophy. Mr. N is referred for noninvasive cardiac evaluation, including a treadmill exercise test (ETT) and a transthoracic echocardiogram. On the ETT, he reaches a peak heart rate of 170 beats/min during exercise and has to terminate the test because of severe dyspnea at a workload of 7 METS. (METS are metabolic equivalents, a measure of energy consumption; a value of 7 METS is below normal for this patient’s age.) His blood pressure at peak exercise is 240/120 mm Hg. There is no evidence of myocardial ischemia by ECG criteria. The two-dimensional echocardiogram reveals concentric-pattern left ventricular hypertrophy, an enlarged left atrium, and normal aortic and mitral valves. Global and regional left ventricular systolic function are normal. Left ventricular diastolic filling is abnormal, with a reduced rate of early rapid filling and a significant increase in the extent of filling during atrial systole.
Questions
1. What are the current recommendations for initiation of antihypertensive drug therapy, and what are the therapeutic goals?
2. Thiazide diuretics have been used for many years as first-line therapy in patients with hypertension. What specific clinical circumstances might favor use of another agent, such as an angiotensin converting enzyme inhibitor?
3. Given the severity of hypertension in this case, Mr. N will likely require at least two drugs to achieve adequate control of his blood pressure. When is multidrug therapy required?
 PATHOPHYSIOLOGY OF HYPERTENSION
PATHOPHYSIOLOGY OF HYPERTENSION
Hypertension is a widely prevalent disease and a major risk factor for adverse cardiovascular events including stroke, coronary artery disease, peripheral vascular disease, heart failure, and chronic kidney disease. In primary prevention studies, there is a continuous relationship between blood pressure and adverse cardiovascular outcomes including death. Although elevated diastolic blood pressure had long been the main indication for initiating antihypertensive treatment, it is now appreciated that elevated systolic blood pressure alone (isolated systolic hypertension) is also sufficient indication for treatment, particularly in elderly patients. Recommendations for treatment of hypertension have recently undergone revision, with differential treatment thresholds based on age and presence of chronic kidney disease or diabetes (Fig. 26-1). The current recommendations reflect the results of recent randomized trials, which did not show a benefit of intensive blood pressure control in some populations and which also found that patients with chronic kidney disease or diabetes are at higher risk of cardiovascular events.
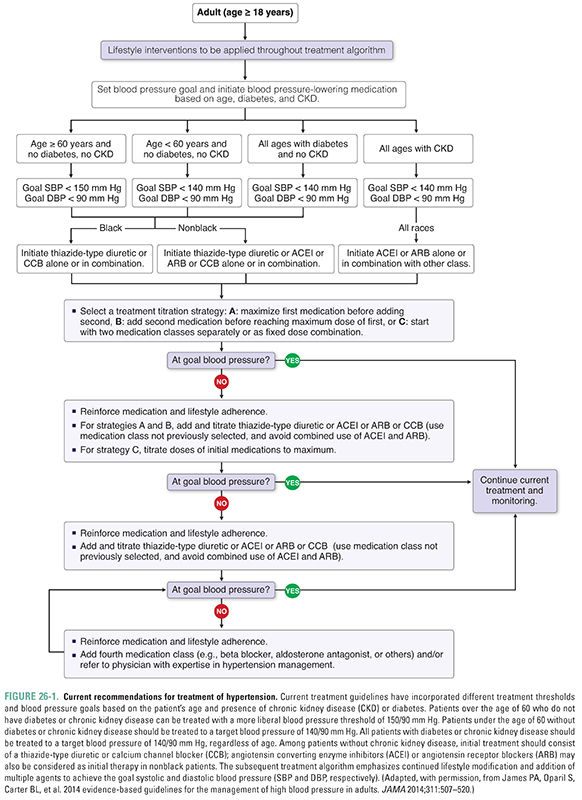
One of the main obstacles in the treatment of hypertension is the largely asymptomatic nature of the disease, even in patients with marked elevation in systemic blood pressure. This disconnect between symptoms and long-term adverse consequences has earned hypertension the designation “silent killer.” For example, Mr. N began to exhibit symptoms only after exercising. Nonetheless, the severity of his hypertension puts him at major risk for developing coronary artery disease, stroke, and heart failure. Thus, effective strategies for detection and management of hypertension are critical elements in the primary and secondary prevention of cardiovascular disease.
Fortunately, the number and spectrum of agents available to treat patients with hypertension have expanded dramatically over the past two decades. These drugs can be administered initially as single agents (monotherapy). However, the progressive nature of hypertension characteristically leads to the use of a multidrug regimen. Although the clinical endpoints of therapy can vary somewhat from patient to patient, the principal goal of treatment is to reduce the measured blood pressure, typically to levels less than 140 mm Hg systolic and less than 90 mm Hg diastolic.
Hypertension is typically categorized as either primary (essential) or secondary hypertension. Essential hypertension, in which the cause of the elevation in blood pressure is unknown, affects 90–95% of the hypertensive population. The etiology of essential hypertension is likely multifactorial, including both genetic factors and environmental factors such as alcohol use, obesity, and salt consumption. A more complete understanding of the pathophysiology of primary hypertension awaits the elucidation of underlying genetic predispositions and/or molecular mechanisms. Secondary hypertension refers to patients in whom elevated blood pressure can be attributed to a defined cause. Examples of secondary hypertension include primary hyperaldosteronism, oral contraceptive use, intrinsic renal disease, and renovascular disease.
The principal determinants of blood pressure are discussed in Chapter 22, Pharmacology of Vascular Tone. Briefly, blood pressure is determined by the product of heart rate, stroke volume, and systemic vascular resistance (Fig. 26-2). Heart rate is determined largely by sympathetic activity. Stroke volume depends on loading conditions (preload and afterload) and contractility. Systemic vascular resistance reflects the aggregate vascular tone of the arteriolar subdivisions of the systemic circulation. A rational pharmacologic approach to the treatment of both primary and secondary hypertension requires an understanding of the physiology of normal blood pressure regulation and the mechanisms that could be responsible for hypertension in individual patients.
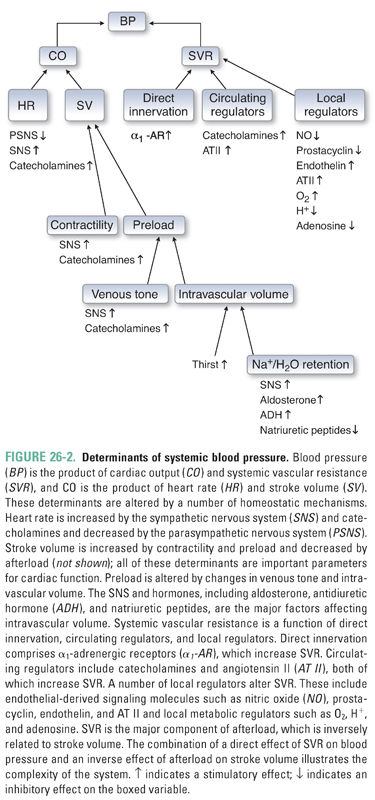
One potential mechanism for persistent blood pressure elevation is a primary elevation in cardiac output (“high-output” hypertension). A “hyperkinetic” circulation can result from excessive sympathoadrenal activity and/or increased sensitivity of the heart to basal levels of neurohumoral regulators. The hemodynamic pattern of pump-based hypertension (i.e., increased cardiac output [CO] with normal systemic vascular resistance [SVR]) is most often seen in younger patients with essential hypertension; this pattern can evolve over time into a hemodynamic profile in which the principal locus of disease appears to shift to the peripheral vasculature (see below). The underlying mechanism of high-output hypertension makes treatment with β-adrenoceptor antagonists attractive in this population.
Vascular resistance-based hypertension (i.e., normal CO with increased SVR) is a common mechanism underlying hypertension in the elderly. In individuals with this form of hypertension, it is hypothesized that the vasculature is abnormally responsive to sympathetic stimulation, circulating factors, or local regulators of vascular tone. The abnormal responsiveness of the vasculature may be mediated in part by endothelial damage or dysfunction, which is known to disrupt the normal equilibrium between local vasodilatory (e.g., nitric oxide) and vasoconstrictive (e.g., endothelin) factors. In addition, ion channel defects in vascular smooth muscle can cause abnormal elevations in basal vasomotor tone that result in increased systemic vascular resistance. Vascular resistance-based hypertension may present as a predominant elevation of systolic blood pressure. Studies have demonstrated the effectiveness of thiazide diuretics in this population, making such agents the preferred initial treatment.
Abnormalities of renal function can also contribute to the development of systemic hypertension. Excessive Na+ and H2O retention by the kidney is responsible for volume-based hypertension. Renal parenchymal disease, caused by glomerular injury with reduction of functional nephron mass and/or excessive secretion of renin, can lead to an abnormal increase in intravascular volume. Alternatively, ion channel mutations can impair normal Na+ excretion. Renovascular disease (e.g., renal artery stenosis caused by atherosclerotic plaques, fibromuscular dysplasia, emboli, vasculitis, or external compression) can result in decreased renal blood flow. In response to this decrease in perfusion pressure, juxtaglomerular cells increase the secretion of renin, which in turn leads to increased production of angiotensin II and aldosterone. The latter mediators increase both vasomotor tone and Na+ and H2O retention, leading to a hemodynamic profile in which both CO and SVR are elevated.
Dysfunction of the neuroendocrine system—including abnormal central regulation of basal sympathetic tone, atypical stress responses, abnormal responses to signals from baroreceptors and intravascular volume receptors, and excessive production of hormones that act to regulate the circulation—can alter cardiac, vascular, and/or renal function, leading to increased systemic blood pressure. Examples of endocrine abnormalities associated with systemic hypertension include excessive secretion of catecholamines (pheochromocytoma), excessive secretion of aldosterone by the adrenal cortex (primary aldosteronism), and excessive production of thyroid hormones (hyperthyroidism).
 CLINICAL MANAGEMENT OF HYPERTENSION
CLINICAL MANAGEMENT OF HYPERTENSION
As discussed above, hypertension presents a complex clinical challenge, since blood pressure elevation may be asymptomatic for many years even as substantial end-organ damage occurs. As a result, the effective treatment of hypertension requires strategies to identify asymptomatic patients, especially those at high risk for the adverse end-organ effects of the disease. Because antihypertensive drugs can add inconvenience to the life of a patient who is asymptomatic, long-term treatment of the hypertensive patient requires the use of drug regimens that are individualized for optimal adherence and efficacy. This requires consideration of safety profile, dosing schedule, and cost.
The first line in hypertension treatment is counseling regarding the importance of lifestyle modifications. Lifestyle modifications associated with favorable results in hypertensive patients include weight loss, increased physical activity, smoking cessation, and a low-fat, low-sodium diet. Reduction or elimination of exogenous agents that can induce hypertension—such as ethanol, oral contraceptives, glucocorticoids, and stimulant drugs—can also have demonstrable clinical benefit. While nonpharmacologic therapies alone may not achieve a sufficient reduction in blood pressure, they remain critical adjuncts to pharmacologic treatment.
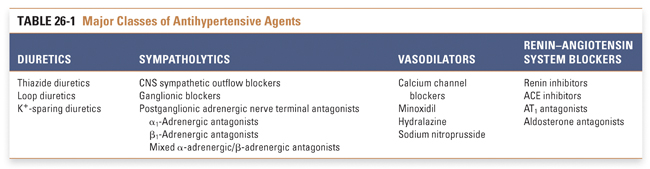
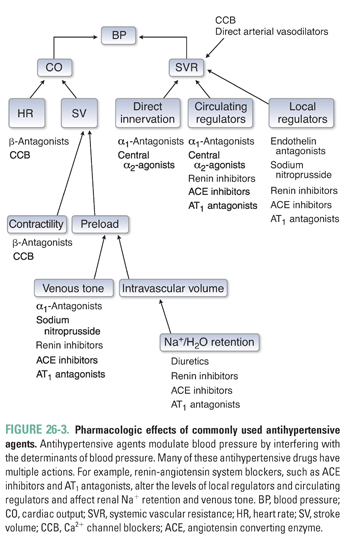
An extensive armamentarium of drugs is used to treat systemic hypertension. Ultimately, though, these agents all exert their effects on blood pressure through a reduction in cardiac output and/or systemic vascular resistance. Strategies currently used to treat hypertension include reduction of intravascular volume with concomitant vasodilation (diuretics), down-regulation of sympathetic tone (β-antagonists, α1-antagonists, central sympatholytics), modulation of vascular smooth muscle tone (calcium channel blockers, K+ channel openers), and inhibition of the neurohumoral regulators of the circulation (renin inhibitors, ACE inhibitors, AT1 antagonists [angiotensin II type 1 receptor antagonists]) (Table 26-1 and Fig. 26-3). The reduction in blood pressure caused by these agents is sensed by baroreceptors and renal juxtaglomerular cells, which can activate counter-regulatory responses that attenuate the magnitude of blood pressure reduction. These compensatory responses can be substantial, necessitating dose adjustments and/or the use of more than one agent to achieve long-term blood pressure control (Fig. 26-4).
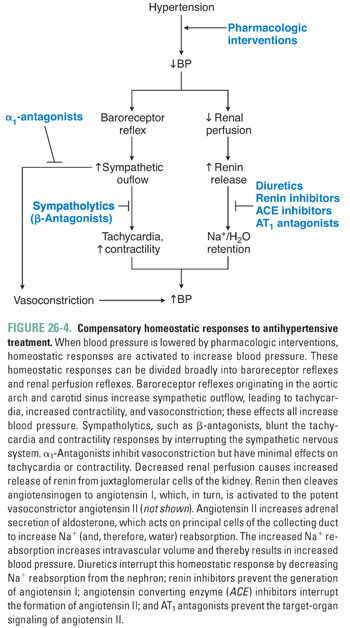
Reduction of Intravascular Volume
Although diuretics have long been a cornerstone of antihypertensive therapy, the mechanism of action of diuretics in hypertension is incompletely understood. As discussed in Chapter 21, Pharmacology of Volume Regulation, diuretics decrease intravascular volume by increasing renal excretion of Na+ and H2O. However, volume depletion alone is unlikely to fully explain the antihypertensive effect of diuretics.
Thiazide diuretics (e.g., hydrochlorothiazide) are the natriuretic drugs most commonly prescribed for the treatment of hypertension (Table 26-2). The pharmacokinetic and pharmacodynamic characteristics of the thiazides make them especially useful agents in the treatment of chronic hypertension. Thiazides have high oral availability and long duration of action. The initial antihypertensive effect seems to be mediated by decreasing intravascular volume. Therefore, thiazides are particularly effective in patients with volume-based hypertension, such as patients with primary renal disease and African American patients. Thiazides induce an initial decrease in intravascular volume that decreases blood pressure by lowering cardiac output. However, the decrease in cardiac output stimulates the renin-angiotensin system, which leads to volume retention and attenuation of the effect of the thiazide on volume status. It is hypothesized that a vasodilatory effect of the thiazides complements the compensated volume depletion, leading to a sustained decrease in blood pressure. This hypothesis is supported by the observation that the maximal antihypertensive effect of the thiazides is frequently achieved at doses lower than those needed to achieve a maximal diuretic effect. Therefore, thiazides achieve their blood pressure effect by influencing both cardiac output and systemic vascular resistance.
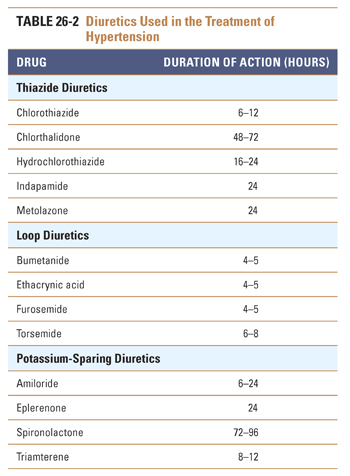
The Joint National Commission (JNC) treatment algorithm suggests thiazide diuretics as a potential first-line agent for many patients and a preferred first-line agent for African American patients (Fig. 26-1). This recommendation arises from the results of large-scale trials, which found favorable outcomes and decreased cost associated with thiazide therapy. The practice at present is to initiate thiazide therapy at low doses (e.g., 12.5–25 mg/day).
Loop diuretics (e.g., furosemide) are infrequently prescribed for the treatment of mild or moderate hypertension. These agents typically have a relatively short duration of action (4–6 hours), and despite the brisk diuresis that follows their administration, their antihypertensive efficacy is often modest. It is thought that this modest impact on blood pressure is due to activation of compensatory responses involving the neurohumoral regulators of intravascular volume and systemic vascular resistance. There are, however, several well-recognized clinical situations in which loop diuretics are preferable to thiazides, including malignant hypertension (see below) and volume-based hypertension in patients with advanced chronic kidney disease.
K+-sparing diuretics (e.g., spironolactone, triamterene, amiloride) are less efficacious than thiazide and loop diuretics and are used primarily in combination with other diuretics to attenuate or correct drug-induced kaliuresis (K+ excretion) and the resultant hypokalemia. An exception is spironolactone, an aldosterone receptor antagonist that is especially effective in the treatment of secondary hypertension caused by hyperaldosteronism. Hypokalemia is a common metabolic adverse effect of the thiazide and loop diuretics, which inhibit Na+ reabsorption in proximal segments of the nephron and thereby increase delivery of Na+ and water to distal segments of the nephron. Increased distal Na+ delivery results in a compensatory increase in Na+ reabsorption in the distal tubule, which is coupled to an increase in K+ excretion. Because the latter effect is mediated by aldosterone (see Chapter 21), the K+-sparing diuretics attenuate this effect and thereby help to maintain normal serum potassium levels. It should be emphasized that both ACE inhibitors (which decrease aldosterone activity and K+ excretion) and K+ supplements may need to be decreased or eliminated in patients taking K+-sparing diuretics, because life-threatening hyperkalemia has been reported in association with clinical use of the K+-sparing agents. Use of these agents should be undertaken with great caution in patients with even mild degrees of renal insufficiency.
Down-Regulation of Sympathetic Tone
Drugs that modulate adrenergic activity are discussed in detail in Chapter 11, Adrenergic Pharmacology; refer to that chapter for descriptions of the tissue distribution of α- and β-adrenergic receptors and the cardiovascular effects mediated by these receptors. Sympatholytic drugs treat hypertension via two major mechanisms: reduction of systemic vascular resistance and/or reduction of cardiac output. Clinically, these agents are broadly divided into β-adrenoceptor antagonists, α-adrenoceptor antagonists, and central sympatholytics.
β-Adrenoceptor antagonists (e.g., propranolol, metoprolol, atenolol, nebivolol) are commonly prescribed agents in the treatment of hypertension. The negative chronotropic and inotropic effects of these agents (and the reductions in heart rate, stroke volume, and cardiac output that follow) account for the initial antihypertensive effect of the β-antagonists. Decreased vasomotor tone, with a consequent decrease in systemic vascular resistance, has also been reported with longer term therapy.
The β-antagonist-induced reduction in vasomotor tone may seem paradoxical, given that β2-adrenergic receptors in the peripheral vasculature mediate vasodilation. However, antagonism of β1-adrenergic receptors in the kidney decreases secretion of renin and thereby decreases production of the potent vasoconstrictor, angiotensin II. The latter effect likely predominates, even when nonselective β-receptor antagonists are administered. Although β-antagonists effectively reduce blood pressure in hypertensive patients, these agents typically do not cause hypotension in individuals with normal blood pressure. Increased baseline sympathetic activity in hypertensive patients may in part explain the efficacy of β-antagonists in lowering blood pressure in these individuals. In contrast, basal activation of β-receptors in normal individuals may be sufficiently low that receptor antagonists have little hemodynamic effect. β-Antagonist therapy has been associated with both elevation of serum triglyceride levels and reduction of high-density lipoprotein (HDL) levels; the clinical significance of these potentially harmful metabolic effects remains unclear. Noncardiac adverse effects of β-antagonist therapy may include exacerbation of glucose intolerance (hyperglycemia), sedation, impotence, depression, and bronchoconstriction.
Mixed α–β antagonists (e.g., labetalol) are available in both oral and parenteral formulations. Intravenous administration of labetalol causes a substantial reduction in blood pressure and has found wide use in the treatment of hypertensive emergencies. Oral labetalol is also used in the long-term treatment of hypertension. One potential advantage of this drug is that the decrease in blood pressure achieved by reduction of systemic vascular resistance (because labetalol antagonizes α1-receptors) is not associated with the reflex increase in heart rate or cardiac output that can occur when pure vasodilator drugs are used as monotherapy (because labetalol also antagonizes cardiac β1-receptors).
In recent years, β-adrenoceptor antagonists have been used less frequently in the initial treatment of hypertension, due to clinical data suggesting that they may not be as efficacious as diuretics or inhibitors of the renin-angiotensin-aldosterone system. However, these agents are still important in the treatment of hypertension when there are other clinical indications for a β-adrenoceptor antagonist, such as coronary artery disease or heart failure. β-Receptor antagonists are generally efficacious in the treatment of hypertension in younger patients.
α1-Adrenergic antagonists (e.g., prazosin, terazosin, doxazosin) are also used in the treatment of high blood pressure. α1-Adrenergic antagonists inhibit peripheral vasomotor tone, reducing vasoconstriction and decreasing systemic vascular resistance. The absence of adverse effects on the serum lipid profile during long-term treatment with α1-adrenergic antagonists is often cited as a distinctive advantage of these agents relative to other antihypertensive medications. However, the long-term benefit of this advantage, if any, remains to be determined in randomized clinical trials. Furthermore, in a large trial comparing different antihypertensives, an increased incidence of heart failure was observed in the group randomized to doxazosin.
Nonselective α-adrenergic antagonists (e.g., phenoxybenzamine, phentolamine) are not employed in the long-term treatment of hypertension because excessive compensatory responses can result from their long-term use. For example, antagonism of central α2-adrenergic receptors disinhibits sympathetic outflow, resulting in unopposed reflex tachycardia. However, these agents are indicated for the medical treatment of pheochromocytoma.
The α2-adrenergic agonists methyldopa, clonidine, and guanabenz reduce sympathetic outflow from the medulla, leading to decreases in heart rate, contractility, and vasomotor tone. These drugs are available in oral formulations (clonidine is also available as a transdermal patch) and were widely used in the past despite their unfavorable adverse effect profile. The availability of multiple alternative agents, as well as the current trend toward the use of multidrug regimens at submaximal doses, have substantially diminished the clinical role of α2-agonists in the treatment of hypertension.
Ganglionic blockers (e.g., trimethaphan, hexamethonium) inhibit nicotinic cholinergic activity at sympathetic ganglia. These agents are extremely effective at lowering blood pressure. However, the severe adverse effects of combined parasympathetic and sympathetic blockade (e.g., constipation, blurred vision, sexual dysfunction, and orthostatic hypotension) have made ganglionic blockers of historic interest only.
Some sympatholytic agents (e.g., reserpine, guanethidine) are taken up into the terminals of postganglionic adrenergic neurons, where they induce long-term depletion of neurotransmitter from norepinephrine-containing synaptic vesicles (see Chapter 11). These agents lower blood pressure by decreasing the activity of the sympathetic nervous system. However, reserpine and guanethidine have little role in the contemporary treatment of hypertension because of their significant adverse-effect profiles, which include severe depression (reserpine) and orthostatic hypotension and sexual dysfunction (guanethidine).
Modulation of Vascular Smooth Muscle Tone
As discussed in Chapter 22, vascular tone is dependent on the degree of vascular smooth muscle contraction. Vasodilators reduce systemic vascular resistance by acting on arteriolar smooth muscle and/or the vascular endothelium. The major mechanisms of action of the arterial vasodilators include blockade of Ca2+ channels and opening of metabotropic K+ channels.
Ca2+ channel blockers (e.g., verapamil, diltiazem, nifedipine, amlodipine) are oral agents that are widely used in the long-term treatment of hypertension. Calcium channel blockers (CCBs) have a variety of hemodynamic effects, reflecting the multiple sites at which calcium is involved in the electrical and mechanical events of the cardiac cycle and in vascular regulation. These agents can act as arterial vasodilators, negative inotropes, and/or negative chronotropes. The dihydropyridine agents nifedipine and amlodipine act primarily as vasodilators. In contrast, the nondihydropyridine drugs verapamil and diltiazem act principally as negative inotropes and chronotropes, thereby decreasing myocardial contractility, heart rate, and impulse conduction. Thus, CCBs can lower blood pressure through reduction of both systemic vascular resistance and cardiac output. CCBs are often used in combination with other cardioactive drugs, either as components of a multidrug antihypertensive regimen or for combined antihypertensive and antianginal treatment in patients with ischemic heart disease (IHD).
Given the distinctive pharmacodynamic effects of the different CCBs, the potential adverse effects of CCB therapy (including adverse interactions with other cardiovascular therapies) are agent specific. The nondihydropyridine agents verapamil and diltiazem should be used with caution in patients who have impaired left ventricular (LV) systolic function, as these agents can exacerbate systolic heart failure (see below). These agents should also be used with caution in patients with conduction system disease, as these drugs can potentiate functional abnormalities of the sinoatrial (SA) and atrioventricular (AV) nodes. Both of these cautions are particularly relevant in patients receiving concomitant β-antagonist therapy.
Minoxidil and hydralazine are orally available arterial vasodilators that are occasionally used in the long-term treatment of hypertension. Minoxidil is a metabotropic K+ channel opener that hyperpolarizes vascular smooth muscle cells and thereby attenuates the cellular response to depolarizing stimuli. Hydralazine is a less powerful vasodilator with an uncertain mechanism of action. Both minoxidil and hydralazine can cause compensatory retention of Na+ and H2O as well as reflex tachycardia; these adverse effects are more frequent and more severe with minoxidil than with hydralazine. Concomitant use of a β-antagonist and a diuretic can mitigate these adverse effects. The use of hydralazine is limited by the frequent occurrence of tolerance and tachyphylaxis to the drug. In addition, increases in the total daily dose of hydralazine can be associated with a drug-induced lupus syndrome. Given the more favorable safety profile of the Ca2+ channel blockers, the use of minoxidil is now largely restricted to patients with severe hypertension that is refractory to other pharmacologic therapies. Of note, hydralazine (in combination with isosorbide dinitrate) has now emerged as an adjunctive therapy (i.e., in patients who are already receiving an ACE inhibitor and a β-antagonist) in the treatment of systolic heart failure in African American patients.
Modulation of the Renin-Angiotensin-Aldosterone System
Renin-angiotensin-aldosterone system blockers include the renin inhibitor aliskiren, the ACE inhibitors (e.g., captopril, enalapril, lisinopril), and the angiotensin receptor (AT1) antagonists (e.g., losartan, valsartan). These agents are increasingly used in the treatment of hypertension.
Aliskiren is a competitive inhibitor of renin, the enzyme that cleaves angiotensinogen to angiotensin I. This early-stage blockade of the renin-angiotensin-aldosterone system may theoretically result in more effective reduction of blood pressure and regression of left ventricular hypertrophy than that achieved by angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Recent clinical trials have suggested that increased renal failure and hypotension may occur when aliskiren is prescribed in combination with ACE inhibitors or ARBs. For this reason, aliskiren is less commonly used in the treatment of hypertension.
Angiotensin Converting Enzyme Inhibitors
ACE inhibitors prevent the ACE-mediated conversion of angiotensin I to angiotensin II, leading to decreased circulating levels of angiotensin II and aldosterone. By decreasing angiotensin II levels, ACE inhibitors decrease systemic vascular resistance and thereby decrease the impedance to LV ejection. By decreasing aldosterone levels, these agents promote natriuresis and thereby reduce intravascular volume. ACE inhibitors also decrease bradykinin degradation, and the resulting increase in circulating bradykinin causes vasodilation. ACE inhibitors are effective in patients with hyper-reninemic hypertension, but these agents also reduce blood pressure in patients with low-to-normal circulating renin levels. The antihypertensive effectiveness of ACE inhibitors in patients with low-to-normal plasma renin activity may be due to potentiation of the vasodilatory effects of bradykinin, although this hypothesis is unproven.
Therapy with ACE inhibitors is as effective as therapy with thiazide diuretics or β-antagonists in the treatment of hypertension. ACE inhibitors are attractive antihypertensive agents because these drugs seem to have unique benefits (e.g., a decrease in the loss of renal function in patients with chronic kidney disease) and relatively few adverse effects (ACE inhibitors do not increase the risk of hypokalemia or cause elevated serum glucose or lipid levels). Despite these attractive features, it merits emphasis that, in at least one large comparison trial, thiazide diuretics were more cardioprotective than ACE inhibitors.
ACE inhibitors should be administered with caution in patients with intravascular volume depletion. Such patients may have reduced renal perfusion at baseline, leading to a compensatory increase in renin and angiotensin II; this increase in angiotensin II is one of the physiologic mechanisms by which glomerular filtration rate (GFR) is maintained in the face of relative renal hypoperfusion. Administration of ACE inhibitors to such patients can disrupt this autoregulatory mechanism, leading to renal insufficiency. The same autoregulatory mechanism is the basis for the contraindication to ACE inhibitors in patients with bilateral renal artery stenosis (or unilateral stenosis in patients with a single kidney). Despite these cautionary notes, it should be emphasized that ACE inhibitors are considered the preferred therapy in the hypertensive diabetic patient, as these agents have been shown to delay the onset and progression of diabetic glomerular disease through favorable effects on intraglomerular pressure.
AT1 Antagonists (Angiotensin Receptor Blockers)
Angiotensin II receptor (AT1) antagonists (also known as angiotensin receptor blockers, or ARBs) are oral antihypertensive agents that competitively antagonize the binding of angiotensin II to its cognate AT1 receptors; examples include losartan, valsartan, and irbesartan. In addition to their antihypertensive effect, these agents may also reduce reactive arteriolar intimal proliferation. Like ACE inhibitors, AT1 antagonists are effective in lowering blood pressure and are sometimes substituted for ACE inhibitors in patients with ACE inhibitor-induced cough. Cough, a common adverse effect of ACE inhibitor therapy, results from drug-induced increases in bradykinin levels; this effect often leads to nonadherence or discontinuation of the drug. Because AT1 antagonists do not affect the activity of the converting enzyme responsible for bradykinin degradation, cough is not an adverse effect of therapy with ARBs.
Monotherapy (treatment with a single drug) is often sufficient to normalize blood pressure in patients with mild hypertension; this approach may improve patient adherence and avoid the risk of potential drug interactions. Controversy exists as to which antihypertensive agents are preferred as initial therapy. Thiazide diuretics, ACE inhibitors, AT1 antagonists, and calcium channel blockers (CCBs) are similar in terms of efficacy in lowering blood pressure (each effectively lowers blood pressure in 30–50% of patients). Ultimately, the ideal agent is that which reduces a patient’s blood pressure to the optimal range with the least severe adverse effects. Drug toxicities are often related to drug dose, and therefore, the clinician must also consider the use of a combination of “synergistic” agents at lower doses, especially if blood pressure control is marginal or inadequate.
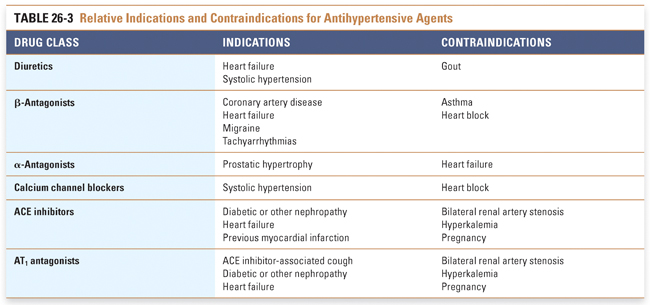
Certain clinical circumstances favor initiating a specific class of antihypertensive medication (Table 26-3). β-Antagonists are the agents of choice in patients with a history of myocardial infarction (MI). ACE inhibitors are recommended in patients with left ventricular dysfunction, diabetes, and/or chronic kidney disease. Diuretics are effective in treating hypertension associated with volume retention in nephrotic syndrome. ACE inhibitors are also used in nephrotic syndrome to attenuate the degree of proteinuria.
Stepped care refers to the progressive, step-by-step addition of drugs to a therapeutic regimen for hypertension. Combination therapy is based on the use of agents with distinct mechanisms of action; it also emphasizes the use of submaximal doses of drugs in an attempt to minimize potential adverse effects and toxicities.
Two examples of combination therapy are the use of an ACE inhibitor with either a diuretic or a calcium channel blocker. These combinations have several potential mechanistic advantages. By inducing a mild degree of volume depletion, thiazide diuretics activate the renin-angiotensin system. If this response is blocked by an ACE inhibitor, then the antihypertensive effect of the thiazide is potentiated. Furthermore, inhibition of the renin-angiotensin system by itself promotes natriuresis. Finally, the combination of a thiazide and an ACE inhibitor decreases systemic vascular resistance.
When an ACE inhibitor is used with a calcium channel blocker, the combination may have an additive effect on regression of left ventricular hypertrophy. The addition of a calcium channel blocker may also potentiate ACE inhibitor-mediated peripheral vasodilation. A recent study (ACCOMPLISH trial) has suggested that the combination of an ACE inhibitor with a calcium channel blocker may reduce the incidence of cardiovascular events more than the combination of an ACE inhibitor with a thiazide diuretic, despite similar reductions in blood pressure.
Certain classes of antihypertensive drugs have been reported to be more effective than others in special populations. Some data also suggest that distinct etiologies of hypertension may be more or less prevalent in different populations.
Elderly patients tend to respond more favorably to diuretics and dihydropyridine Ca2+ channel blockers than to other antihypertensive agents. β-Antagonists are more likely to cause SA or AV node dysfunction or to impair myocardial function in elderly patients; these effects are likely related to the higher prevalence of conduction system disease and LV systolic dysfunction in such patients. Elderly patients also tend to have decreased circulating levels of renin and have been reported to be less responsive to ACE inhibitors.
Hypertension in patients of African descent seems to be more responsive to diuretics and Ca2+ channel blockers than to β-antagonists and ACE inhibitors. (A notable exception is the favorable response of young African Americans to β-antagonist therapy.) Reports indicate that some African Americans may have lower circulating renin levels, and this could account for the observation that ACE inhibitors are less effective in these patients. Reports have also suggested that the prevalence of Na+ sensitivity is substantially increased in some African Americans, including both the hypertensive and the normotensive cohort. Although less well studied, there is some evidence of differential responsiveness to the various classes of antihypertensive agents in hypertensive Asian and Hispanic cohorts.
Despite these demographic observations, the clinical benefit of drug selection on the basis of differential responsiveness to specific drug classes has not been evaluated systematically. For example, although elderly patients are reportedly less responsive to β-antagonists, the results of the Systolic Hypertension in the Elderly Project (SHEP Trial) indicate that both β-antagonists and diuretics are, in fact, associated with mortality reduction, and this favorable treatment effect is demonstrated within several years of treatment initiation. Similarly, although reports have suggested that African Americans are less responsive to β-antagonists and ACE inhibitors, it would be difficult to apply these observations to the treatment of a hypertensive, diabetic African American with chronic kidney disease or to advocate for the use of a thiazide diuretic in a hypertensive African American with a history of previous MI. Finally, it should again be emphasized that the risk of disease complications related to hypertension cannot be explained by the degree of blood pressure elevation alone. Conversely, the full spectrum of treatment benefits cannot be explained by the degree of blood pressure reduction alone. For these reasons, the empirical observation that some antihypertensive agents do not lower blood pressure as effectively in some patients does not necessarily mean that these drugs will be less effective in preventing future cardiovascular disease morbidity and mortality in these patients. These questions remain the focus of active research.
The term hypertensive crisis refers to clinical syndromes characterized by severe (typically acute) elevations in blood pressure. This abrupt increase in blood pressure can cause acute vascular injury and derivative end-organ damage. Although most cases of severe hypertension were, at one time, designated as “hypertensive crisis” or “malignant hypertension,” current practice attempts to distinguish those patients in whom the blood pressure elevation and vascular injury are acute (hypertensive emergency) from the patient cohort in which the temporal course of blood pressure elevation is more gradual and the end-organ damage is chronic and slowly progressive.
A true hypertensive emergency is a life-threatening condition in which severe and acute blood pressure elevation is associated with acute vascular injury. The vascular injury can manifest clinically as retinal hemorrhages, papilledema, encephalopathy, and acute (or acute superimposed on chronic) renal insufficiency; this syndrome is often associated with acute left ventricular failure. The pathogenesis of malignant hypertension remains unclear. However, it is likely that fibrinoid arteriolar necrosis contributes to the signs and symptoms of this syndrome. Fibrinoid arteriolar necrosis of specific vascular beds can result in acute vascular injury and end-organ hypoperfusion (e.g., renal failure, stroke). Fibrinoid arteriolar necrosis can also lead to microangiopathic hemolytic anemia.
Treatment of patients with hypertensive emergency necessitates rapid reduction of blood pressure to prevent end-organ damage. Drug classes used to treat this condition include parenteral vasodilators (e.g., clevidipine, nitroprusside, fenoldopam, nicardipine), diuretics (e.g., furosemide), and/or β-antagonists (e.g., labetalol). Because of the acuity of the syndrome and the need to titrate these powerful antihypertensive agents carefully, patients are hospitalized for treatment. After the acute episode has been controlled, subsequent lowering of blood pressure to the normal range of the patient is then attempted more cautiously over a longer period of time (12–24 hours), in an effort to decrease the risk of critical-organ hypoperfusion and extension of vascular injury.
Although malignant hypertension is a life-threatening medical emergency, it is an uncommon expression of hypertensive disease that occurs in far less than 1% of hypertensive patients. More common are cases of hypertensive urgency, in which the blood pressure elevation is less acute and the target organ disease has been present for some time. Conditions illustrative of hypertensive urgency include a stroke or MI that is accompanied by severe blood pressure elevation or acute left heart failure with severe hypertension.

PART II: ISCHEMIC HEART DISEASE
Mr. N is treated for hypertension with low-dose hydrochlorothiazide and an ACE inhibitor. He returns for follow-up visits at 1 month and 6 months, and reports that he is doing well. He faithfully adheres to his prescribed medical regimen and notes a definite improvement in exercise capacity. His regular blood pressure measurements now show readings of 130 to 150/86 to 90 mm Hg. A serum lipid profile is notable for increased total cholesterol, with a moderately elevated LDL. Low-dose aspirin is added to his regimen. Treatment with a lipid-lowering agent is also advised, but Mr. N declines, instead requesting that his lipid profile be rechecked after a period of diet and lifestyle modifications.
An exercise tolerance test 1 year after his initial visit is notable for improved exercise capacity (10 MET workload), with blunting of the heart rate and reduction of the blood pressure at peak exercise compared to the original study (120/min and 190/90 mm Hg, respectively); there is no evidence of myocardial ischemia by ECG criteria. A repeat LDL cholesterol determination is within the normal range (128 mg/dL). His medications (aspirin, hydrochlorothiazide, and ACE inhibitor) are continued, and routine follow-up is established.
One week later, Mr. N experiences the abrupt onset of severe retrosternal chest pressure. He is visibly diaphoretic and dyspneic. He calls 911 and is transported to the local emergency department, where an ECG shows sinus tachycardia and ST segment elevation in the inferior leads. Emergency cardiac catheterization is performed, confirming total occlusion of a dominant right coronary artery, and percutaneous coronary intervention (PCI) is performed with placement of a coronary stent. The procedure is successful, and he remains free of chest pain and is hemodynamically stable. ECG and serum enzyme changes (peak creatine kinase [CK], 2,400 IU/L [normal, 60–400 IU/L]; cardiac isoform [MB] fraction, positive) are consistent with an evolving myocardial infarction. A repeat echocardiogram immediately before Mr. N’s discharge from the hospital demonstrates concentric left ventricular hypertrophy with a left ventricular ejection fraction of 40% (normal, >55%); the inferior wall from the base to the apex is akinetic, with thinning of the myocardium in this akinetic region.
Questions
4. Which class of lipid-lowering agent is appropriate for this patient?
5. Which pharmacologic interventions are appropriate during the interval between the patient’s emergency department evaluation and his cardiac catheterization?
6. What are the critical drug components of a postmyocardial infarction treatment regimen in the setting of left ventricular dysfunction?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


