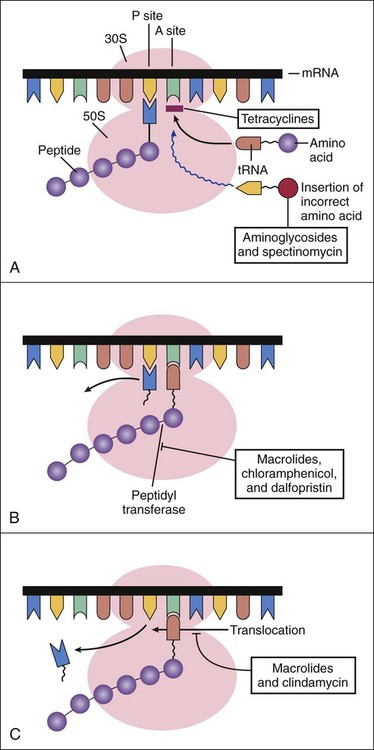
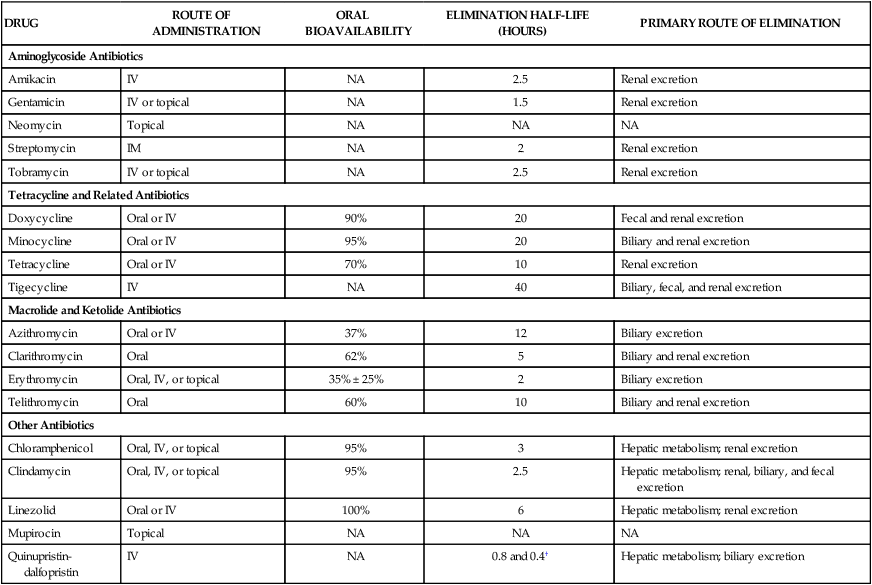
After the introduction of penicillin, scientists began an extensive search for antibiotics that could inhibit penicillin-resistant bacteria. A number of Streptomyces species were isolated from soil samples collected from all over the world, and these species eventually yielded several new classes of antibiotics, including the aminoglycosides, tetracyclines, and macrolides. Streptomycin, an aminoglycoside, was the first new antibiotic to be introduced through these efforts (Box 39-1). The basic steps in bacterial protein synthesis are illustrated in Figure 39-1. These steps include the binding of aminoacyl transfer RNA (tRNA) to the ribosome, the formation of a peptide bond, and translocation. Aminoacyl tRNA binds to the 30S ribosomal subunit, whereas peptide bond formation and translocation involve components of the 50S ribosomal subunit. As shown in Figure 39-1, each type of antibiotic discussed in this chapter acts at a specific site on the ribosome to inhibit one or more steps in protein synthesis. Tetracyclines and aminoglycosides act at the 30S ribosomal subunit. Macrolides, chloramphenicol, dalfopristin, and clindamycin act at the 50S ribosomal subunit. The aminoglycosides include amikacin, gentamicin, neomycin, streptomycin, and tobramycin. The properties and major clinical uses of these drugs are compared in Tables 39-1 and 39-2. TABLE 39-1 Pharmacokinetic Properties of Bacterial Protein Synthesis Inhibitors* IM, Intramuscular; IV, intravenous; NA, not applicable. *Values shown are the mean of values reported in the literature. †The half-lives for quinupristin and dalfopristin are 0.8 and 0.4 hours, respectively. The drugs are given in combination. TABLE 39-2 Major Clinical Uses of Selected Bacterial Protein Synthesis Inhibitors
Inhibitors of Bacterial Protein Synthesis
Overview
Bacterial Protein Synthesis
Sites of Drug Action
Drugs That Affect the 30S Ribosomal Subunit
Aminoglycosides
DRUG
ROUTE OF ADMINISTRATION
ORAL BIOAVAILABILITY
ELIMINATION HALF-LIFE (HOURS)
PRIMARY ROUTE OF ELIMINATION
Aminoglycoside Antibiotics
Amikacin
IV
NA
2.5
Renal excretion
Gentamicin
IV or topical
NA
1.5
Renal excretion
Neomycin
Topical
NA
NA
NA
Streptomycin
IM
NA
2
Renal excretion
Tobramycin
IV or topical
NA
2.5
Renal excretion
Tetracycline and Related Antibiotics
Doxycycline
Oral or IV
90%
20
Fecal and renal excretion
Minocycline
Oral or IV
95%
20
Biliary and renal excretion
Tetracycline
Oral or IV
70%
10
Renal excretion
Tigecycline
IV
NA
40
Biliary, fecal, and renal excretion
Macrolide and Ketolide Antibiotics
Azithromycin
Oral or IV
37%
12
Biliary excretion
Clarithromycin
Oral
62%
5
Biliary and renal excretion
Erythromycin
Oral, IV, or topical
35% ± 25%
2
Biliary excretion
Telithromycin
Oral
60%
10
Biliary and renal excretion
Other Antibiotics
Chloramphenicol
Oral, IV, or topical
95%
3
Hepatic metabolism; renal excretion
Clindamycin
Oral, IV, or topical
95%
2.5
Hepatic metabolism; renal, biliary, and fecal excretion
Linezolid
Oral or IV
100%
6
Hepatic metabolism; renal excretion
Mupirocin
Topical
NA
NA
NA
Quinupristin-dalfopristin
IV
NA
0.8 and 0.4†
Hepatic metabolism; biliary excretion
DRUG
INFECTIONS
Streptomycin
Plague, tularemia, drug-resistant tuberculosis
Gentamicin, tobramycin, and amikacin
Infective endocarditis; infections with aerobic gram-negative bacilli, including Pseudomonas aeruginosa
Tetracycline antibiotics
Lyme disease, Rocky Mountain spotted fever, ehrlichiosis, granuloma inguinale, brucellosis, cholera, relapsing fever, peptic ulcer disease from Helicobacter pylori, chlamydial urethritis, acne, MRSA
Tigecycline
Skin and soft tissue infections with MRSA; intraabdominal infections with various organisms; community-acquired pneumonia
Erythromycin
Respiratory tract infections with streptococci, pneumococci, Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae
Azithromycin, clarithromycin
Respiratory tract infections with organisms sensitive to erythromycin, Haemophilus influenzae, Moraxella catarrhalis, Mycobacterium avium-intracellulare
Clarithromycin
Peptic ulcer disease from H. pylori
Telithromycin
Community-acquired pneumonia from pneumococci, Legionella, Chlamydia, and other organisms
Chloramphenicol
Meningitis, brain abscess
Clindamycin
Streptococcal, staphylococcal, and anaerobic infections
Quinupristin-dalfopristin
Skin and soft tissue infections with Staphylococcus aureus or Streptococcus pyogenes; infections with Enterococcus faecium
Linezolid
Infections with vancomycin-resistant E. faecium, streptococci, and methicillin-resistant staphylococci
Mupirocin
Impetigo from streptococci, staphylococci; eradication of nasal colonization of MRSA
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree