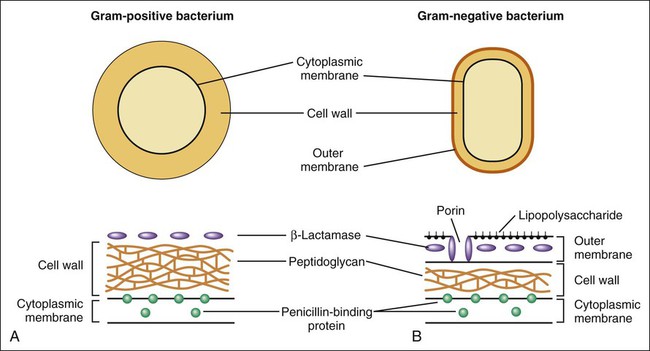
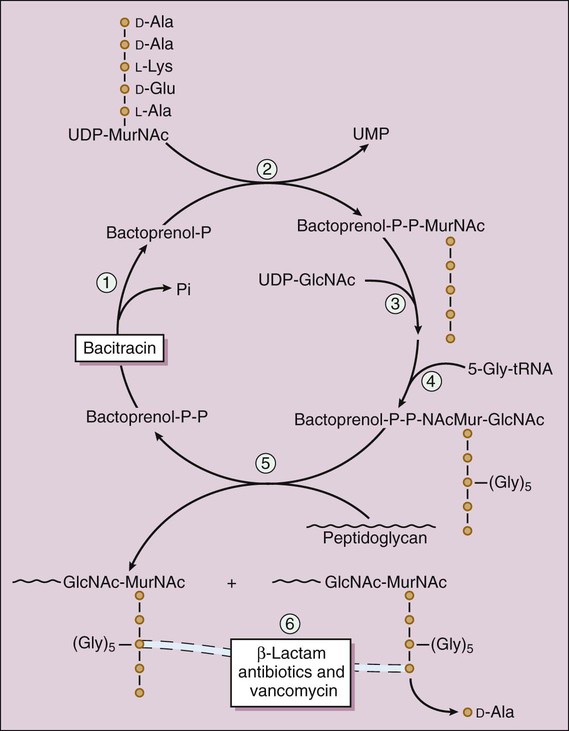
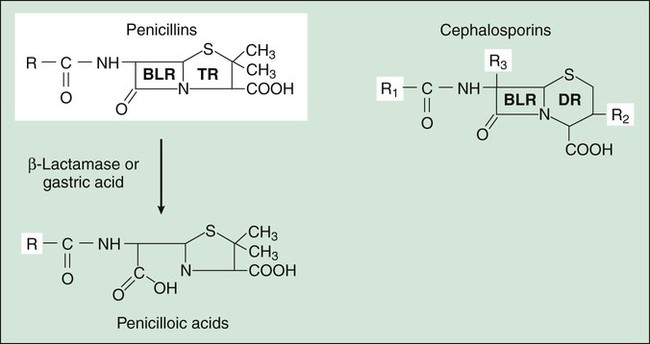
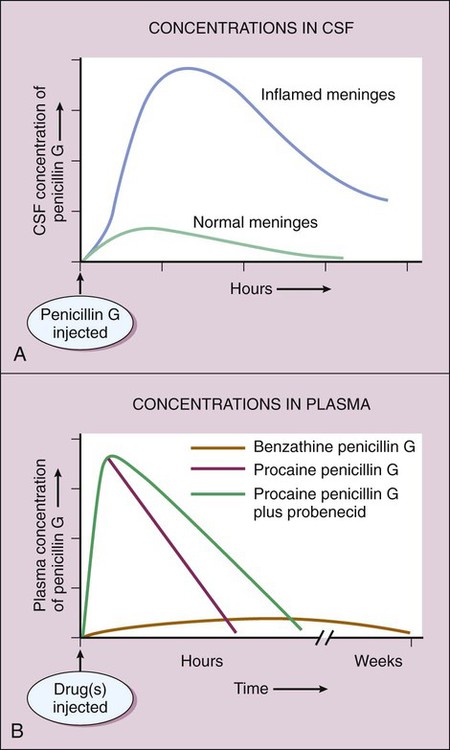
Two components of the cell envelope that are found in both gram-positive and gram-negative bacteria are the cytoplasmic membrane and the cell wall. The cell wall is much thicker in gram-positive bacteria than it is in gram-negative bacteria. The envelope of each gram-negative bacterium also has an outer membrane that is not found in other types of bacteria. The cell envelope components are illustrated in Figure 38-1. The bacterial cytoplasmic membrane is the target of two peptide antibiotics, daptomycin and polymyxin. These drugs act directly on the cell membranes to increase membrane permeability and thereby cause the cytoplasmic contents to leak out of the cell. The properties and uses of these antibiotics are discussed in Chapter 40. The cell wall consists primarily of peptidoglycan, a polymer constructed from repeating disaccharide units of N–acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc). Each disaccharide is attached to others through glycosidic bonds. Each molecule of MurNAc has a peptide containing two molecules of D-alanine and a pentaglycine side chain (Fig. 38-2). The strands of peptidoglycan in the cell wall are cross-linked by a transpeptidase reaction in which the glycine pentapeptide of one strand is attached to the penultimate D-alanine molecule of another strand. During this reaction, the terminal D-alanine is removed. The β-lactam antibiotics form a covalent bond with PBPs and thereby inhibit the catalytic activity of these enzymes. Inhibition of some PBPs prevents elongation or cross-linking of peptidoglycan (see Fig. 38-2), whereas inhibition of other PBPs leads to the bacterium’s autolysis or to its change to a spheroplast or a filamentous form. Bacitracin and fosfomycin inhibit cell wall peptidoglycan synthesis by blocking specific steps in the formation of the disaccharide precursor, MurNAc-GlcNAc. As shown in Figure 38-2, bacitracin inhibits the dephosphorylation of bactoprenol pyrophosphate, which is the carrier lipid required for regeneration of bactoprenol phosphate, the active carrier of the disaccharide precursor. Fosfomycin inhibits enolpyruvyl transferase, the enzyme that catalyzes the condensation of uridine diphosphate–GlcNAc (UDP-GlcNAc) with phosphoenolpyruvate to synthesize UDP-MurNAc. Vancomycin binds tightly to the D-alanyl-D-alanine portion of the peptidoglycan precursor and prevents bonding of the penultimate D-alanine to the pentaglycine peptide during cross-linking of peptidoglycan strands. The penicillins consist of a β-lactam ring fused to a thiazolidine ring to which a unique chemical structure (R group) is attached for each antibiotic (Fig. 38-3). The natural penicillins isolated from strains of Penicillium were originally assigned letter designations because their chemical structures could not be identified at that time. Penicillin G and penicillin V are the only natural penicillins still used today, and they are classified as narrow-spectrum drugs. Semisynthetic penicillins are produced by substituting a different R group for the R group of natural penicillin. The penicillinase-resistant penicillins have a large, bulky R group that protects them from hydrolysis by staphylococcal penicillinase, a specific type of bacterial β-lactamase. The penicillins are widely distributed to organs and tissues except the central nervous system. Because penicillins readily penetrate the cerebrospinal fluid when the meninges are inflamed (Fig. 38-4), they can be administered intravenously for the treatment of meningitis. Most penicillin antibiotics are eliminated primarily by active renal tubular secretion and have short half-lives of about 0.5 to 1.3 hours (Table 38-1). A few penicillins (e.g., ampicillin and nafcillin) are excreted primarily in the bile. The renal tubular secretion of penicillins is inhibited by probenecid, a drug that competes with penicillins for the organic acid transporter located in the proximal tubule. Probenecid has been used to slow the excretion and prolong the half-life of penicillin G (see Fig. 38-4). TABLE 38-1 Pharmacokinetic Properties of Selected Bacterial Cell Wall Synthesis Inhibitors*
Inhibitors of Bacterial Cell Wall Synthesis
Overview
Cell Envelope
Cytoplasmic and Outer Membranes
Cell Wall
Sites of Drug Action
β-Lactam Drugs
Other Drugs
β-Lactam Antibiotics
Penicillins
Chemistry
Pharmacokinetics
DRUG
ROUTE OF ADMINISTRATION
ELIMINATION HALF-LIFE (HOURS)
PRIMARY ROUTE OF ELIMINATION
β-Lactam Antibiotics
Narrow-Spectrum Penicillins
Penicillin G
Oral or parenteral
0.5
Renal (TS)
Penicillin V
Oral
1.0
Renal (TS)
Penicillinase-Resistant Penicillins
Dicloxacillin
Oral
0.6
Renal (TS)
Nafcillin
Oral or parenteral
0.5
Biliary
Extended-Spectrum Penicillins
Amoxicillin
Oral
1.0
Renal (TS)
Ampicillin
Oral or parenteral
1.0
Renal (TS) and biliary
Piperacillin and ticarcillin
Parenteral
1.2 to 1.3
Renal (TS)
First-Generation Cephalosporins
Cefazolin
Parenteral
2.0
Renal (TS)
Cephalexin
Oral
0.5
Renal (TS)
Second-Generation Cephalosporins
Cefotetan
Parenteral
4.0
Renal (TS)
Cefoxitin
Parenteral
0.8
Renal (TS)
Cefprozil
Oral
1.3
Renal (TS)
Cefuroxime
Oral or parenteral
1.7
Renal (TS)
Third-, Fourth-, and Advanced-Generation Cephalosporins
Cefdinir
Oral
1.7
Renal (TS)
Cefotaxime
Parenteral
1.6†
Renal (TS)
Ceftaroline
Parenteral (IV)
2.6
Renal (GF)
Ceftazidime
Parenteral
1.8
Renal (GF)
Ceftriaxone
Parenteral
8.0
Biliary
Cefepime
Parenteral
2.0
Metabolized
Monobactam
Aztreonam
Parenteral
1.7
Metabolized
Carbapenems
Parenteral
1.0 to 1.2
Renal (TS)
Other Bacterial Cell Wall Synthesis Inhibitors
Bacitracin
Topical
NA
NA
Fosfomycin
Oral
6.0
Renal (GF)
Vancomycin
Oral or parenteral
6.0
Renal (GF) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Inhibitors of Bacterial Cell Wall Synthesis
Only gold members can continue reading. Log In or Register to continue