Chapter 10
Infection
Neal S. Rote and Sue E. Huether
For most of human history infectious disease was the primary disease-related cause of death; bubonic/pneumonic plague (The Black Death) of the 14th century killed more than 50 million people worldwide, the Spanish influenza of 1918 to 1919 killed between 50 and 100 million people within 2 years, and it was not uncommon for frequent regional outbreaks of diseases such as smallpox or cholera to kill hundreds of thousands of people.1 Modern health care has shown great progress in preventing and treating infectious diseases through the success of public health initiatives, vaccination programs, and the use of antibiotics. The greatest progress has occurred in developed countries where death from infections is most common among those with debilitating diseases, nutritional deficiencies, or immunosuppression. Death from influenza and pneumonia was the eighth leading cause of death in the United States in 2011.2 As a result of these initiatives, smallpox has been eradicated from the globe (the last reported case was in 1975 in Somalia), measles is almost eradicated in the Western Hemisphere, and many diseases, such as tuberculosis and polio, are on the decline. Although the advent of sanitary living conditions, clean water, uncontaminated food, vaccinations, and antimicrobials had improved the health in many countries, infectious disease remains a significant threat to life in many parts of the world, including India, Africa, and Southeast Asia. The impact of differences in economic prosperity is significant.2a The leading causes of death in high-income countries were heart disease, stroke, and other cerebrovascular diseases; cancers of the respiratory tract; and Alzheimer disease and other dementias, with lower respiratory tract infections (influenza and pneumonia) the sole infectious cause in the top 10. In low-income countries, where the implementation of programs to prevent infection has been less effective, 6 of the top 10 causes of death are caused by infection: lower respiratory tract infections (#1), diarrheal diseases (#2), HIV/AIDS (#3), malaria (#5), tuberculosis (#7), and neonatal infections (#10).
• Vast and rapid urbanization in many areas of the world, resulting in a breakdown in public health programs and a more rapid spread of infection
• Poverty and social inequality
• Global travel, allowing more rapid spread of disease from isolated areas to virtually any point around the world in a few hours
• Globalization of the food supply
• Human encroachment into wilderness areas, resulting in contact with previously sequestered infectious agents
• Practice of prescribing antibiotics excessively or not taking antibiotics for a complete course of therapy, or, even when appropriately used, facilitates emergence of antibiotic-resistant microorganisms
• Decreases in federal research budgets to study infectious disease
• Denial of a problem by governments, allowing infections to spread in an uncontrolled way
• Diminished use of effective insecticides
• Increased global warming, allowing insect vectors to spread into and breed in areas that were previously too cool for them
Emerging Infections
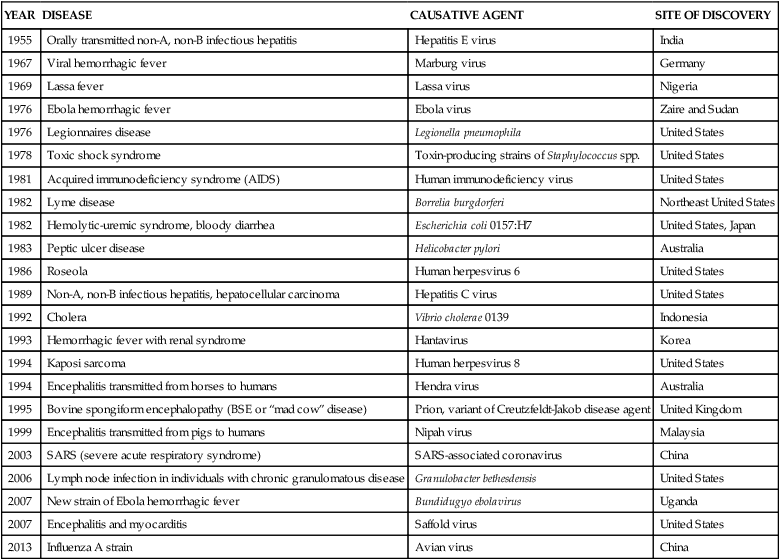
The emergence of previously unknown infections is not a new event in human history. However, the current rate may be unprecedented.3 Within 1 generation, more than 40 previously unknown infections have arisen, and some examples are presented in Table 10-1. Several have extremely high mortality rates of more than 50% including severe acute respiratory syndrome (SARS) (in those older than 65 years), Ebola virus, Marburg virus, “mad cow” disease, Nipah virus (up to 75%), and acquired immunodeficiency syndrome (AIDS) (almost 100% in untreated persons). However, most either spread very slowly (e.g., AIDS) or initially appear in relatively isolated areas and are effectively controlled by quarantine (e.g., Ebola virus). Although none of these infections has developed into the worldwide scourges portrayed in the Hollywood movies Andromeda Strain and I Am Legend, the potential of reversion to more rapidly spreading variants is a concern of public health agencies worldwide.
TABLE 10-1
EXAMPLES OF EMERGENT INFECTIONS
| YEAR | DISEASE | CAUSATIVE AGENT | SITE OF DISCOVERY |
| 1955 | Orally transmitted non-A, non-B infectious hepatitis | Hepatitis E virus | India |
| 1967 | Viral hemorrhagic fever | Marburg virus | Germany |
| 1969 | Lassa fever | Lassa virus | Nigeria |
| 1976 | Ebola hemorrhagic fever | Ebola virus | Zaire and Sudan |
| 1976 | Legionnaires disease | Legionella pneumophila | United States |
| 1978 | Toxic shock syndrome | Toxin-producing strains of Staphylococcus spp. | United States |
| 1981 | Acquired immunodeficiency syndrome (AIDS) | Human immunodeficiency virus | United States |
| 1982 | Lyme disease | Borrelia burgdorferi | Northeast United States |
| 1982 | Hemolytic-uremic syndrome, bloody diarrhea | Escherichia coli 0157:H7 | United States, Japan |
| 1983 | Peptic ulcer disease | Helicobacter pylori | Australia |
| 1986 | Roseola | Human herpesvirus 6 | United States |
| 1989 | Non-A, non-B infectious hepatitis, hepatocellular carcinoma | Hepatitis C virus | United States |
| 1992 | Cholera | Vibrio cholerae 0139 | Indonesia |
| 1993 | Hemorrhagic fever with renal syndrome | Hantavirus | Korea |
| 1994 | Kaposi sarcoma | Human herpesvirus 8 | United States |
| 1994 | Encephalitis transmitted from horses to humans | Hendra virus | Australia |
| 1995 | Bovine spongiform encephalopathy (BSE or “mad cow” disease) | Prion, variant of Creutzfeldt-Jakob disease agent | United Kingdom |
| 1999 | Encephalitis transmitted from pigs to humans | Nipah virus | Malaysia |
| 2003 | SARS (severe acute respiratory syndrome) | SARS-associated coronavirus | China |
| 2006 | Lymph node infection in individuals with chronic granulomatous disease | Granulobacter bethesdensis | United States |
| 2007 | New strain of Ebola hemorrhagic fever | Bundidugyo ebolavirus | Uganda |
| 2007 | Encephalitis and myocarditis | Saffold virus | United States |
| 2013 | Influenza A strain | Avian virus | China |
Concurrently, the incidence and spread of at least 20 previously known infections are increasing. A new strain of cholera that arose in Indonesia in 1961 has spread to Africa and in 1991 to South America. Malaria, dengue fever, and yellow fever are reemerging in areas where they had been eliminated or were unknown. The incidence of tuberculosis is increasing in countries that had reported declines and has risen by almost 33% between the mid-1980s and early 1990s. Diphtheria has reemerged as a major health issue in Russia. In 1994 plague was reported in India after being dormant for a generation. War has led to outbreaks of Marburg hemorrhagic fever during civil war in Angola during 1975 to 2002 and cholera in the Democratic Republic of the Congo among Rwandan refugees in 1994; about 50,000 refugees died from a combination of cholera and shigella dysentery. The spread of cholera, yellow fever, and epidemic meningococcal disease has rebounded. Decreased insect control programs that were previously successful have led to spread of vector-borne diseases: African trypanosomiasis, dengue hemorrhagic fever, and malaria. Although the United States is relatively free of most of these diseases, the effects of global warming and relaxed control of vectors may result in resurgence. It should not be forgotten that in 1793 a yellow fever outbreak in Philadelphia killed 2000 of the city’s 55,000 inhabitants and forced the U.S. government to abandon the city until the outbreak ceased. To date, outbreaks of locally acquired malaria in the United States have been rare. However, West Nile Virus, also spread by mosquitoes, appeared initially in the Western Hemisphere in 1999 in the New York City area and has spread throughout the continental United States, Canada, and Mexico.4
Microorganisms and Humans: A Dynamic Relationship
For many microorganisms the human body is a hospitable site in which to grow and flourish because of its sufficient nutrients and appropriate conditions of temperature and humidity. In many cases a symbiotic relationship exists, in which both humans and microorganisms benefit (Box 10-1). These microorganisms make up the normal microbiome—the resident microorganisms found in different parts of the body, including the skin, mouth, gastrointestinal tract, respiratory tract, and genital tract5 (see Chapter 7). For instance, the normal bacterial microbiome of the human gut is provided with nutrients from ingested food and in exchange produce enzymes that facilitate the digestion and use of many of the more complex molecules found in the human diet, produce antibacterial factors (e.g., bacteriocins, colicins) that prevent colonization by pathogenic microorganisms, and produce usable metabolites (e.g., vitamin K, B vitamins). This beneficial homeostasis is normally maintained through the physical integrity of the gut and other mechanisms that sequester these microorganisms on the mucosal surface.
Microorganisms and Infections
Process of Infection
The symbiotic relationship with the normal flora can be breached as a result of injury that compromises the physical protective barriers. Damage to the intestinal tract releases intestinal bacteria into the bloodstream, potentially leading to sepsis, shock, and death. Cuts in the skin may allow normally noninfectious bacteria (e.g., S. aureus) to cause local infections (e.g., abscesses, boils) and invade further and infect various organs. Symbiosis is also maintained by the immune and inflammatory systems. If those systems are compromised, many microorganisms will leave their normal sites and cause infection elsewhere in the body. Individuals with immune deficiencies easily become infected with opportunistic microorganisms, which normally would not cause disease but seize the opportunity to do so when a person’s defensive systems are weakened or suppressed (see Chapter 9).
Unlike opportunistic infectious agents, true pathogens have devised means to circumvent the individual’s defenses (discussed in Chapters 7 and 8) and directly cause infection. Successful infection with these agents is usually dependent on adequate numbers of microorganisms rather than compromise of the host’s defenses.
Clinical Infectious Disease
• Incubation period—the period from initial exposure to the infectious agent and the onset of the first symptoms, during which the microorganism has entered the individual, undergone initial colonization, and begun multiplying, but is yet in insufficient numbers to cause symptoms; this period may last from several hours to years
• Prodromal stage—the occurrence of initial symptoms, which are often very mild and include a feeling of discomfort and tiredness
• Invasion period—the pathogen is multiplying rapidly, invading farther and affecting the tissues at the site of initial colonization as well as other areas; the immune and inflammatory responses are being triggered; development of symptoms specifically related to the pathogen and symptoms related to the ongoing protective inflammatory response
• Convalescence—in most instances, the individual’s immune and inflammatory systems have successfully removed the infectious agent, and symptoms decline; alternatively, the disease may be fatal or may enter a latency phase with resolution of symptoms until reactivation at a later time
Fever is not failure of the body to regulate temperature; rather, body temperature is being regulated at a higher level than normal. Body temperature is regulated by nervous system feedback to the hypothalamus, which functions as a central thermostat (see Chapter 16). A large number of agents (pyrogens) can produce fever. In current classification, those pyrogens derived from outside the host are termed exogenous pyrogens and those produced by the individual are termed endogenous pyrogens. There is little evidence that exogenous pyrogens cause fever directly. Such pyrogens indirectly affect the hypothalamus through endogenous pyrogens released by cells of the host. A number of cytokines have been identified as endogenous pyrogens. They are interleukin-1 and interleukin-6 (IL-1 and IL-6, respectively), interferon (IFN), tumor necrosis factor (TNF), and others (see Figure 16-8). These cytokines seem to raise the thermoregulatory set point through stimulation of prostaglandin synthesis and turnover in both thermoregulatory (brain) and nonthermoregulatory (peripheral) tissue. Although it is generally believed that fever has a beneficial value in infection, the molecular mechanism behind the beneficial effects has not been established. Many investigators, however, consider fever as an adaptive host-defense response.
Several factors influence the capacity of a pathogen to cause disease.
• Communicability: The ability to spread from one individual to others and cause disease: measles and pertussis spread very easily; HIV is of lower communicability
• Immunogenicity: The ability of pathogens to induce an immune response
• Infectivity: The ability of the pathogen to invade and multiply in the host
• Mechanism of action: How the microorganism damages tissue
• Pathogenicity: The ability of an agent to produce disease—success depends on communicability, infectivity, extent of tissue damage, and virulence
• Portal of entry: The route by which a pathogenic microorganism infects the host: direct contact, inhalation, ingestion, or bites of an animal or insect
• Toxigenicity: The ability to produce soluble toxins or endotoxins, factors that greatly influence the pathogen’s degree of virulence
• Virulence: The capacity of a pathogen to cause severe disease—for example, measles virus is of low virulence; rabies virus is highly virulent
Infectious diseases are also classified by their prevalence and spread within the community.
• Endemic: Diseases with relatively high, but constant, rates of infection in a particular population
• Epidemic: The number of new infections in a particular population greatly exceeds the number usually observed
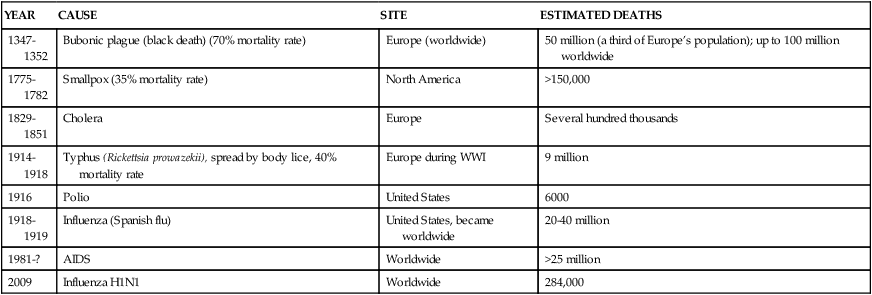
• Pandemic: An epidemic that spreads over a large area, such as a continent or worldwide (Table 10-2)
TABLE 10-2
HISTORIC EXAMPLES OF PANDEMICS
| YEAR | CAUSE | SITE | ESTIMATED DEATHS |
| 1347-1352 | Bubonic plague (black death) (70% mortality rate) | Europe (worldwide) | 50 million (a third of Europe’s population); up to 100 million worldwide |
| 1775-1782 | Smallpox (35% mortality rate) | North America | >150,000 |
| 1829-1851 | Cholera | Europe | Several hundred thousands |
| 1914-1918 | Typhus (Rickettsia prowazekii), spread by body lice, 40% mortality rate | Europe during WWI | 9 million |
| 1916 | Polio | United States | 6000 |
| 1918-1919 | Influenza (Spanish flu) | United States, became worldwide | 20-40 million |
| 1981-? | AIDS | Worldwide | >25 million |
| 2009 | Influenza H1N1 | Worldwide | 284,000 |
Classes of Infectious Microorganisms
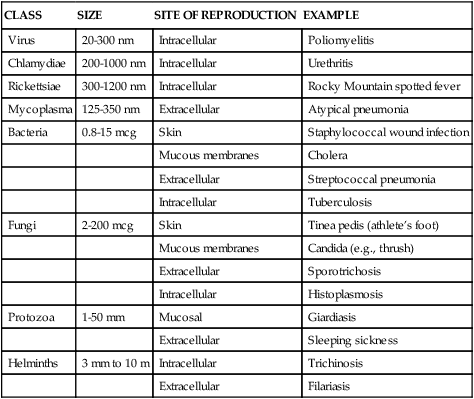
Infectious disease can be caused by microorganisms that range in size from 20 nm (poliovirus) to 10 m (tapeworm). Classes of pathogenic microorganisms and their characteristics are summarized in Table 10-3 and discussed in detail in the following sections.
TABLE 10-3
CLASSES OF ORGANISMS INFECTIOUS TO HUMANS
| CLASS | SIZE | SITE OF REPRODUCTION | EXAMPLE |
| Virus | 20-300 nm | Intracellular | Poliomyelitis |
| Chlamydiae | 200-1000 nm | Intracellular | Urethritis |
| Rickettsiae | 300-1200 nm | Intracellular | Rocky Mountain spotted fever |
| Mycoplasma | 125-350 nm | Extracellular | Atypical pneumonia |
| Bacteria | 0.8-15 mcg | Skin | Staphylococcal wound infection |
| Mucous membranes | Cholera | ||
| Extracellular | Streptococcal pneumonia | ||
| Intracellular | Tuberculosis | ||
| Fungi | 2-200 mcg | Skin | Tinea pedis (athlete’s foot) |
| Mucous membranes | Candida (e.g., thrush) | ||
| Extracellular | Sporotrichosis | ||
| Intracellular | Histoplasmosis | ||
| Protozoa | 1-50 mm | Mucosal | Giardiasis |
| Extracellular | Sleeping sickness | ||
| Helminths | 3 mm to 10 m | Intracellular | Trichinosis |
| Extracellular | Filariasis |
Bacterial Infection
• “True bacteria” divide by binary fission and may have a variety of morphologies, including cocci (spherical), bacilli (rod shaped), vibrios (comma-shaped rods), or spirilla (twisted, rod shaped). Most disease-causing bacteria fall into this classification.
• Filamentous bacteria may have branching, mycelium-like structures that resemble fungi. Examples include the mycobacteria Mycobacterium tuberculosis and Mycobacterium leprae that respectively cause tuberculosis and leprosy.
• Spirochetes are flexible spiral filaments that are motile. Most are anaerobic. Pertinent examples include Borrelia recurrentis (relapsing fever), T. pallidum (syphilis), and Borrelia burgdorferi (Lyme disease).
• Mycoplasma lack a rigid cell wall and are small and pleomorphic. They are the smallest and most simple members of the bacteria. Mycoplasma pneumoniae causes atypical pneumonia, and Mycoplasma genitalium is a suspected cause of urethritis and pelvic inflammatory disease.
• Rickettsia are strict intracellular parasites that can be rod-shaped, spherical, or pleomorphic. They are typically spread by insect vectors and cause Rocky Mountain spotted fever (Rickettsia rickettsii) and typhus (Rickettsia prowazekii).
• Chlamydia are also strict intracellular parasites, but with more complex intracellular life cycles. The primary chlamydial pathogen is Chlamydia trachomatis, which causes the most common bacterial sexually transmitted infection (pelvic inflammatory disease) and eye infections (conjunctivitis).
Bacteria are also categorized as gram-negative or gram-positive. Gram-negative bacteria do not retain crystal violet dye in the Gram-staining process whereas gram-positive bacteria do retain crystal violet dye. Gram-negative bacteria also have a lipopolysaccharide (LPS) coat in the outer membrane, which consists of lipid A, a core polysaccharide, and O antigen. The LPS coat is also known as endotoxin (see Figure 10-1 and further discussion on p. 306). Common bacterial pathogens are listed in Table 10-4.
TABLE 10-4
| MICROORGANISM | GRAM STAIN | RESPIRATORY PATHWAY | INTRACELLULAR OR EXTRACELLULAR |
| Respiratory Tract Infections | |||
| Upper Respiratory Tract Infections | |||
| Corynebacterium diphtheriae (diphtheria) | Gram + | Facultative anaerobic | Extracellular |
| Haemophilus influenzae | Gram − | Facultative anaerobic | Extracellular |
| Streptococcus pyogenes (group A) | Gram + | Facultative anaerobic | Extracellular |
| Otitis Media | |||
| Haemophilus influenzae | Gram − | Facultative anaerobic | Extracellular |
| Moraxella catarrhalis | Gram − | Aerobic | Extracellular |
| Streptococcus pneumoniae | Gram + | Facultative anaerobic | Extracellular |
| Lower Respiratory Tract Infections | |||
| Bacillus anthracis (pulmonary anthrax) | Gram + | Facultative anaerobic | Extracellular |
| Bordetella pertussis (whooping cough) | Gram − | Aerobic | Extracellular |
| Chlamydia pneumoniae | Not stainable | Aerobic | Obligate intracellular |
| Escherichia coli | Gram − | Facultative anaerobic | Extracellular |
| Haemophilus influenzae | Gram − | Facultative anaerobic | Extracellular |
| Klebsiella pneumoniae | Gram − | Facultative anaerobic | Extracellular |
| Legionella pneumophila | Gram − | Aerobic | Facultative intracellular |
| Mycobacterium tuberculosis | Weak gram + | Aerobic | Extracellular |
| Mycoplasma pneumoniae | Not stainable | Aerobic | Extracellular |
| Neisseria meningitidis (develops into meningitis) | Gram − | Aerobic | Extracellular |
| Pseudomonas aeruginosa | Gram − | Aerobic | Extracellular |
| Streptococcus agalactiae (group B; develops into meningitis) | Gram + | Facultative anaerobic | Extracellular |
| Streptococcus pneumoniae | Gram + | Facultative anaerobic | Extracellular |
| Yersinia pestis (plague) | Gram − | Facultative anaerobic | Extracellular |
| Gastrointestinal Tract Infections | |||
| Inflammatory Gastrointestinal Tract Infections | |||
| Bacillus anthracis (gastrointestinal anthrax) | Gram + | Facultative anaerobic | Extracellular |
| Clostridium difficile | Gram + | Anaerobic | Extracellular |
| Escherichia coli 0157:H7 | Gram − | Facultative anaerobic | Extracellular |
| Vibrio cholerae | Gram − | Facultative anaerobic | Extracellular |
| Vibrio parahaemolyticus | Gram − | Facultative anaerobic | Extracellular |
| Invasive Gastrointestinal Tract Infections | |||
| Brucella abortus (brucellosis, undulant fever leading to sepsis, heart infection) | Gram − | Aerobic | Intracellular |
| Campylobacter jejuni | Gram − | Microaerophilic | Extracellular |
| Francisella tularensis | Gram − | Strict anaerobic | Facultative intracellular |
| Helicobacter pylori (gastritis and peptic ulcers) | Gram − | Microaerophilic | Extracellular |
| Listeria monocytogenes (leading to sepsis and meningitis) | Gram + | Aerobic | Intracellular |
| Salmonella typhi (typhoid fever) | Gram − | Anaerobic | Extracellular |
| Shigella sonnei | Gram − | Facultative anaerobic | Extracellular |
| Food Poisoning | |||
| Bacillus cereus | Gram + | Facultative anaerobic | Extracellular |
| Clostridium botulinum | Gram + | Anaerobic | Extracellular |
| Clostridium perfringens | Gram + | Anaerobic | Extracellular |
| Staphylococcus aureus | Gram + | Facultative anaerobic | Extracellular |
| Sexually Transmitted Infections | |||
| Chlamydia trachomatis (pelvic inflammatory disease) | Not stainable | Aerobic | Intracellular |
| Neisseria gonorrhoeae (urethritis) | Gram − | Aerobic | Facultative intracellular |
| Treponema pallidum (spirochete; syphilis) | Gram − | Aerobic | Extracellular |
| Skin and Wound Infections | |||
| Bacillus anthracis (cutaneous anthrax) | Gram + | Facultative anaerobic | Extracellular |
| Borrelia burgdorferi (Lyme disease; spirochete) | Gram − | Aerobic | Extracellular |
| Clostridium tetani (tetanus) | Gram + | Anaerobic | Extracellular |
| Clostridium perfringens (gas gangrene) | Gram + | Anaerobic | Extracellular |
| Mycobaterium leprae (leprosy) | Gram + (weakly) | Aerobic | Extracellular |
| Pseudomonas aeruginosa | Gram − | Aerobic | Extracellular |
| Rickettsia prowazekii (rickettsia; typhus) | Gram − | Aerobic | Obligate intracellular |
| Staphylococcus aureus | Gram + | Facultative anaerobic | Extracellular |
| Streptococcus pyogenes (group A) | Gram + | Facultative anaerobic | Extracellular |
| Eye Infections | |||
| Chlamydia trachomatis (conjunctivitis) | Not stainable | Aerobic | Obligate intracellular |
| Haemophilus aegyptus (pink eye) | Gram − | Facultative anaerobic | Extracellular |
| Zoonotic Infections | |||
| Bacillus anthracis (anthrax) | Gram + | Facultative anaerobic | Extracellular |
| Brucella abortus (brucellosis, also called undulant fever) | Gram − | Aerobic | Intracellular |
| Borrelia burgdorferi (spirochete; Lyme disease) | Gram − | Aerobic | Extracellular |
| Listeria monocytogenes | Gram + | Aerobic | Intracellular |
| Rickettsia rickettsii (rickettsia; Rocky Mountain spotted fever) | Gram − | Aerobic | Obligate intracellular |
| Rickettsia prowazekii (rickettsia; typhus) | Gram − | Aerobic | Obligate intracellular |
| Yersinia pestis (plague) | Gram − | Facultative anaerobic | Extracellular |
| Nosocomial Infections | |||
| Enterococcus faecalis | Gram + | Facultative anaerobic | Extracellular |
| Enterococcus faecium | Gram + | Facultative anaerobic | Extracellular |
| Escherichia coli (cystitis) | Gram − | Facultative anaerobic | Extracellular |
| Pseudomonas aeruginosa | Gram − | Obligate anaerobic | Extracellular |
| Staphylococcus aureus | Gram + | Facultative anaerobic | Extracellular |
| Staphylococcus epidermidis | Gram + | Facultative anaerobic | Extracellular |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


