Immunology, Transplantation, and Vaccines
LEARNING OBJECTIVES
IMMUNOLOGY
The human body has a great capacity to resist the many organisms and toxins with which it comes in contact. This defensive mechanism is called immunity and involves an intricate system consisting of both innate and acquired immunity. Innate immunity consists of general processes that are present at birth. These processes are considered the first line of defense against an infectious organism and include skin, gastric acid, mucus, neutrophils, and complement. It is different from acquired immunity in that it is nonspecific, has a fast response, and does not have a memory in response to previous infections.
Active immunity involves humoral and cellular compartments. Although each compartment in the immune system is unique with its own specialized cells, the system works in concert to protect the body from infectious organisms. Active immunity occurs following an initial invasion by a foreign organism or toxin. Each toxin and organism has a unique makeup of proteins or large polysaccharides that differentiates it from other compounds. These proteins and polysaccharides are called antigens.
The fact that not all those exposed to a particular pathogen will develop infection is an important point in infectious disease. The processes involved in the development of infection are likely the virulence of the pathogen and the host susceptibility. In this section we will focus on the components of the immune system that are known to be polymorphic in humans and may contribute to host susceptibility.
Antigen Recognition
B and T cells must have the capacity to recognize and virtually an infinite number of antigens. Both types of lymphocytes have specially adapted receptors for that purpose. The B cell receptor has the same antigen specificity as the antibody it secretes. The T cell receptor is specific and critical for antigen presentation. The genes for these receptors possess unique capacity to undergo deletion, rearrangement, and somatic mutation. The genes for antigen recognition portion of antibodies and T cell receptors are organized in regions. Several copies of the same gene are located within each region. Each copy of the gene is different from the others. These gene cassettes can be rearranged or deleted during the antigen recognition process. In addition, the genes can undergo limited somatic mutation in an effort to create an antibody or T cell receptor that has high affinity for the antigen of interest. These processes allow for the possibility that nearly an infinite number of receptors for antigen recognition can be generated.
Human Leukocyte Antigen
Human leukocyte antigen (HLA) displays an unequaled degree of genetic polymorphism among functional human genes.1 Each individual has HLA genes for class I, HLA-A, HLA-B, and HLA-C, and for class II, HLA-DRB, HLA-DQA, HLA-DQB, HLA-DPA, and HLA-DPB. The HLA genes are located on chromosome 6, and each individual has two alleles for each gene—one set from each parent. The function of HLA is to present peptides from pathogens to T cell receptors to initiate an immune response. HLA is part of the coordinated interaction between antigen-presenting cells and T cells whereby the immune system recognizes self from nonself allowing it to discriminately mount responses to nonself that it recognizes as antigens. The array of possible HLA glycoproteins makes it very unlikely that any pathogen will evade an immune response by all humans. It is also very unlikely that two unrelated humans would have identical HLA genotypes.
HLA class I glycoproteins are ubiquitous and are expressed on the surfaces of every nucleated human cell. They present endogenous peptides derived from the cell itself to cytotoxic T cells. HLA class I glycoproteins play an important role in viral infections. Since viruses use their host cells’ machinery for replication, these cells present viral proteins on their surfaces using HLA class I glycoproteins. The presentation of viral peptides elicits a cell-mediated immune response that destroys the virally infected cell.
HLA class II glycoproteins expressed on an antigen-presenting cell display antigenic peptides derived from the pathogen. A pathogen undergoes phagocytosis by an antigen-presenting cell. The pathogen is digested in a lysosome and digested peptides associated with HLA class II glycoprotein can be presented on the cell surface. A T cell recognizes the antigenic peptide as foreign and initiates an immune response to the antigen.
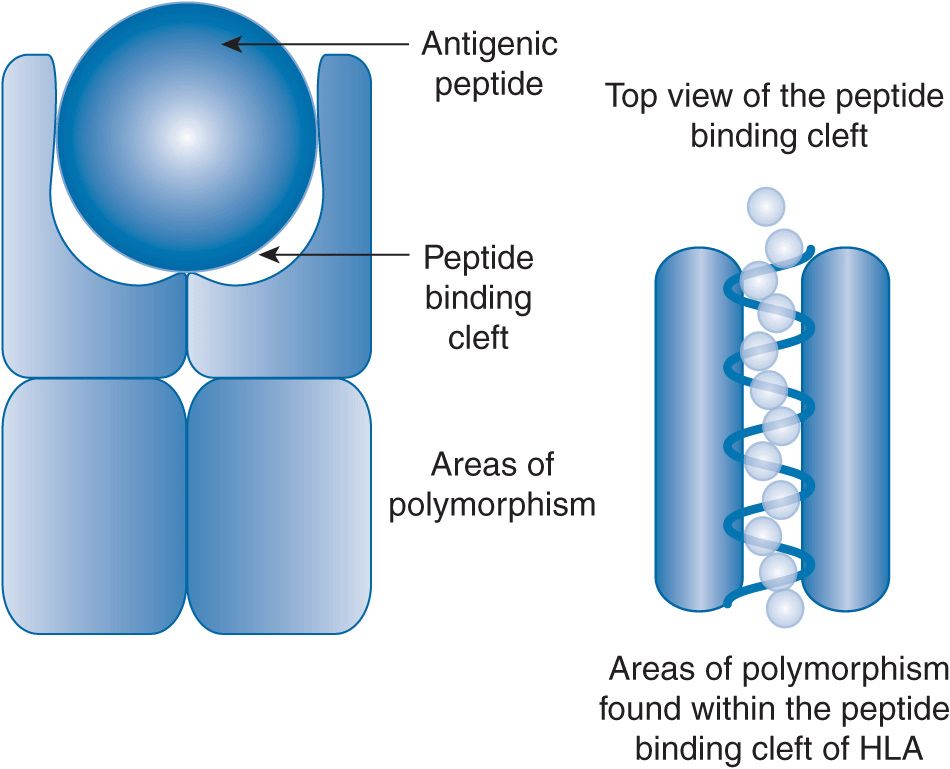
The antigenic peptide must fit into the peptide binding cleft of either HLA class I or class II glycoprotein. Both size and the composition of the peptide determine the fit. For HLA class I glycoproteins, the length of the peptide is typically between 9 and 14 amino acids. For HLA class II, the fit of the antigenic peptide is relatively forgiving as the ends of the peptide binding cleft are open so that the peptide can hang off the ends of the HLA.
The sequence of the antigenic peptide is determined by the pathogen. However, the bulk and charge of the amino acids determine if it will fit in the peptide binding cleft. The polymorphisms within both HLA class I and class II are found almost exclusively in the part of the glycoprotein that makes up the peptide binding cleft (Figure 15–1). Based on the particular surface of the peptide binding cleft, some antigenic peptides may be preferentially presented while others may not be presented at all. The resulting diversity of the HLA peptide binding clefts is advantageous for the survival of the species. The diversity of the peptide binding clefts across the population translates into the ability to recognize and generate an immune response to virtually any pathogen. The type of antigenic peptide that is displayed in the peptide binding cleft is an important factor in the immune response that is generated.
FIGURE 15–1 HLA class II glycoprotein. Two glycoproteins encoded in the HLA region on chromosome 6 form an HLA class 2 glycoprotein. The pocket at the top of the protein is called the peptide binding cleft. The polymorphisms are found in the peptide binding cleft. The top view shows an antigenic peptide in the peptide binding cleft. HLA class II glycoproteins are permissive in their binding as the peptide can hang off the ends of the peptide binding cleft. The polymorphisms found in the peptide binding cleft can change the amino acids that can affect the charge and bulk of the antigenic peptide that can be bound.
HLA and Drug Reaction and Disease Associations
Because of its biology and polymorphisms, a number of disease associations have been made with HLA types. Associations between HLA types and development and progression of autoimmune diseases are well established.2 HLA polymorphisms may contribute to susceptibility to infection.3
Recently, two HLA types have been identified for risk of carbamazepine hypersensitivity reactions. HLA-A*3101 in Europeans and HLA-B*1502 in Asians have been shown to dramatically increase the risk of mild macropapular rash to severe toxic epidermal necrolysis or Stevens–Johnson syndrome.4,5 The risk is important enough that some experts are recommending HLA typing before initiating carbamazepine therapy.4,5
Importantly, the prevalence and frequency of HLA types vary with populations and ethnicities. Clinicians must be cautious when using the literature to evaluate the risk of disease or response due to HLA polymorphisms. The patient under consideration may have to be from a similar ethnic or racial group as the one in which the study was done in order for the association to be applicable.6
Rheumatoid arthritis serves as an example of an autoimmune disease with a well-established connection to HLA polymorphisms. Environmental and other genetic factors contribute to the development of rheumatoid arthritis. Rheumatoid arthritis presents in a wide array of clinical severity. Antibodies to self-proteins are characteristically found in many autoimmune diseases. Anticitrullinated protein antibodies are just one type of autoantibodies that are commonly found in patients with rheumatoid arthritis. Patients with anticitrullinated protein are likely to have rheumatoid arthritis that is more rapidly progressive and severe. HLA-DRB alleles in several ethnic groups have been associated with rheumatoid arthritis. A string of amino acids common to several HLA-DRB alleles confers susceptibility to rheumatoid arthritis with anticitrullinated protein antibodies. The shared epitope contains QKRAA, QRRAA, or RRRAA in the glycoprotein.7 Currently, any HLA-DRB allele that has this shared epitope is believed to increase the risk of rheumatoid arthritis. About one third of the risk for rheumatoid arthritis can be attributed to HLA-DRB gene polymorphisms.8
TRANSPLANTATION
Pharmacogenomics in the field of transplantation is extraordinarily complicated. The genetic basis of tissue antigenicity, immune response, drug metabolism, risk of adverse reactions, and susceptibility to infectious diseases can all be considered. Other chapters cover drug metabolism, adverse events, and infectious diseases in detail.
Tissue typing makes transplantation possible. Blood group is the most basic tissue typing done. ABO blood group antigens are present on most tissues and can be targets of immune response in the recipient. Across-blood-group transplantation is done only in rare circumstances because it is riskier and may involve more intense immunosuppression.
Human Leukocyte Antigen Polymorphisms
HLA matching has been demonstrated to improve outcome in transplantation. Less acute rejection and longer survival is associated with closely matched HLA between recipient and graft. Clinically, matching donor and recipient for HLA-A, HLA-B, and HLA-DR is practical. HLA-C, HLA-DQ, and HLA-DP are antigenic, but it would be impossible to find appropriate matches for these many HLA types. Given the possible combinations, even matching on just HLA-A, HLA-B, and HLA-DR rarely is a six-antigen match made even with the thousands of individuals on the kidney transplant waiting list. However, priority is given to a recipient with a complete match for a kidney. HLA matching for liver transplantation does not improve outcomes.9 A priori HLA matching is not done for lung or heart transplantation. Size matching and severity of illness also factor into the allocation of donor organs. In addition, hearts and lungs do not function as well when subjected to a prolonged ischemia time so the transplant must occur quickly, and the organ cannot be shipped across the country.
Some transplant candidates have preformed antibody responses to HLA. These responses can be initiated by exposure to blood products and are often found in multiparous women. Sometimes, no known risk factor is identified. These anti-HLA antibodies are called panel reactive antibodies (PRA). Traditionally, PRA was measured using a panel of lymphocytes from donors chosen to represent a wide array of HLA types. The PRA was measured by the percentage of lymphocytes from the panel that were killed when mixed with the recipient’s serum. Newer methods take advantage of bead technology and fluorescent labeling. Beads coated with HLA glycoproteins are incubated with the patient’s serum and then with fluorescently labeled antibodies to human antibodies. The degree of coating can be measured by flow cytometry. Individual and clusters of HLA types to which the patient has preformed antibodies can be identified. These preformed antibodies are associated with more rapid graft rejection.
Cytokine Polymorphisms
Production of a number of cytokines known to be important in solid organ transplant rejection is under polymorphic control. Single nucleotide polymorphisms (SNPs) in the gene promoter regions of interferon γ (IFNγ), tumor necrosis factor α (TNFα), interleukin-10 (IL-10), and transforming growth factor β1 (TGF-β1) affect cytokine production. Results of studies linking cytokine gene polymorphisms to transplant graft outcomes have drawn inconsistent conclusions. However, reasonable evidence of an association between high TNFα production and acute rejection of kidney and liver transplants exists.10 This association is biologically plausible because TNFα activates inflammatory cells, increases adhesion molecule expression on vascular endothelium that increases the ability of immune cells to infiltrate the graft, and upregulates the expression of HLA. Some evidence exists that this association is more important when HLA mismatches between recipient and donor are present.11
Chemokine, Costimulation, Growth Factor, and Adhesion Molecule Polymorphisms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree