Public Access to Pharmacogenomic Testing and Patient Counseling
LEARNING OBJECTIVES
INTRODUCTION
There is an eminent need for health care professionals to be prepared to integrate pharmacogenomics into clinical care. There is both growing scientific evidence and an interest of the lay press regarding individualized, tailored, or personalized medicine. As a new area of medicine, pharmacogenomics poses many challenges as well as opportunities. For health care professionals, there are challenges in obtaining a baseline understanding of the relevance of pharmacogenomics and maintaining a current understanding of the evidence supporting pharmacogenomics as it relates to patient care. For patients, there are challenges to being willing to provide genetic information for optimizing their medication therapy and to distinguish the less reputable direct-to-consumer options for genetic testing. Opportunities shared by patients and professionals include an understanding that the “one dose fits all” approach is flawed and that pharmacogenomics is one tool to improve our ability to provide the right drug in the right dose to the right patient.
Despite mounting research, the public’s understanding of pharmacogenomics is not well documented. Individuals may or may not understand the benefits and risks associated with pharmacogenomics. A study published in 2009 by Kobayashi and Satoh assessed the attitudes of 1,103 Japanese adults from the general public (not a patient population) toward pharmacogenomics research and their willingness to donate samples for a DNA biobank to identify genomic markers associated with adverse drug reactions.1 The majority of the respondents showed a positive attitude toward pharmacogenomics research (81%) and donating to a DNA biobank (70.4%). A decreased willingness to donate was significantly associated with older generations. Generally, the respondents had the following concerns regarding a DNA biobank: the confidentiality of their personal information, the manner by which research results were utilized, and the use of their own DNA for research. The authors concluded that a process of public awareness should be put into place to emphasize the beneficial aspects of identifying genomic markers associated with adverse drug reactions and to address the concerns of confidentiality and research raised in their study.1 Although this study was conducted exclusively in Japan, the same concerns are likely to occur in other countries. Health care professionals, including physicians, nurses, pharmacists, and genetic counselors, are best positioned to play a key role in helping the public better understand and interpret pharmacogenomic information.1
HEALTH CARE PROFESSIONAL EDUCATION IN PHARMACOGENOMICS
Health care professionals must be exposed to pharmacogenomics during their education so they can comfortably apply this information in clinical practice and educate their patients. They must have at least a cursory knowledge of genetics and an appreciation for the benefits and limitations to pharmacogenetic tests. Currently, the curricula offered among the health care professional schools, such as schools of nursing, medicine, and pharmacy, vary, but all accredited schools must adhere to a standard group of requirements set by their accrediting body. Pharmacogenomics is not currently a required standard in all educational programs for health care professionals, but many schools offer electives or incorporate some basic concepts and theories into the curriculum.
The 2011 Secretary’s Advisory Committee on Genetics, Health, and Society discovered that health care professionals generally are not comfortable with pharmacogenomics but are excited about the potential opportunities.2 In the report, professional organizations were asked to characterize the need for integrating genetics into the curriculum and training of health care professionals. Although several organizations indicated that it was not a high priority, most felt that this integration is crucial, and several have already implemented national curricula. The 2011 SACGHS survey data found that 70% of responding health care professional organizations view genetics education and training as part of their role or responsibility.
Medical Education
An analysis of the curriculum of medical schools conducted in 2007 found that 77% of programs taught genetics in the first year of medical school, but 47% failed to incorporate any genetic content into the third and fourth years.3 This analysis shows improvement from 2002, when many practicing physicians did not have any genetic instruction during their formal education.4 However, these studies point to a need for medical educators to devote more time to teach pharmacogenomics.
In 2005 the International Society for Pharmacogenomics published a consensus article calling on Deans of Education in medical schools to add pharmacogenomics to their pharmacology curricula.5 It recommended topics that focus on drug response and genetic polymorphisms linked to adverse drug reactions. This call for action spurred 46% of medical schools to offer stand-alone courses with medical genetic content while the remaining 54% embedded this content into existing courses.3
Nursing Education
Much like physicians, the need for education of nurses in genetics and genomics is well documented. A 2005 nursing faculty survey conducted by Prows et al. found that 29% of schools reported no genomic curriculum content.6–8 The most common reasons were already overloaded curricula and lack of knowledge among faculty about genetics. The majority of the programs offered few (less than 5) structured hours on genetic content.8,9 Another article by Prows and Saldana emphasized that nurses must have enough baseline knowledge in genomics to ascertain if a patient’s knowledge is accurate.10 The article lists recommended learning resources, many of which are Web-based training modules or Web sites. Although there is literature citing pharmacogenomics in nursing curricula, it is not consistent across schools.
An editorial published by Van Riper states that it is critical for family nurses to achieve basic competencies in genetics and genomics.11 She emphasizes a leadership role for nurses in gathering genetic data from a family history rather than individual history. Therefore, nursing education must ensure their ability to assess patient knowledge, perception, and response to genomic information and provide additional information to patients with specific needs.
Genetic Counselor Education
Genetic counseling is a relatively young profession. The American Board of Medical Genetics (ABMG) began certifying genetic counselors in 1981 and in 1993 it became part of the American Board of Medical Specialties. At that time the American Board of Genetic Counseling (ABGC) formed out of the ABMG. Since 1993, according to the ABGC Web site, the number of genetic counselors recognized by the ABGC has increased from 495 to more than 2,000 and the number of accredited graduate programs has risen from 18 to 30. As medical science’s understanding of genetics increases, particularly in relation to birth defects and inherited diseases, an increased need for genetic counseling is expected.
In 2006, the National Society of Genetic Counselors’ Task Force Report developed a definition for genetic counseling. In this report, genetic counseling is defined as the process of helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease. This process integrates interpretation of family and medical histories, education about inheritance, testing, management, prevention, resources, research, and counseling.12 The 2008–2009 National Society of Genetic Counselors’ Core Skills Task Force identified six key areas of skills for a successful genetic counselor13:
1. Deep and broad knowledge of genetics
2. Ability to tailor, translate, and communicate complex information in a simple, relevant way for a broad range of audiences
3. Strong interpersonal skills, emotional intelligence, and self-awareness
4. Ability to dissect and analyze a complex problem
5. Research skills (self-education)
6. In-depth knowledge of health care delivery
Genetic counseling is traditionally a clinical service performed by master’s degree-trained professionals educated at accredited genetic counseling training programs. A clinic-based genetic counselor will typically have patient and health care team-oriented skills including communication, critical thinking, counseling, psychosocial assessment, and professional ethics and values. Since their primary purpose is to counsel patients on genetic-related diseases or conditions, current training programs incorporate little medication-related information, pharmacology, or pharmacogenomics.
Pharmacist Education
A report published in 2002 by the Academic Affairs Committee of the American Association of Colleges of Pharmacy identified the need to include curricular outcomes relating to pharmacogenetics and pharmacogenomics in the pharmacy curriculum.14 A literature review from 1960 to 2011 revealed that several schools have research courses, journal clubs, and application-based cases related to pharmacogenomics. Although the content is included in the curriculum, the depth of the material may be limited.15,16
Pharmacists must be formally educated if they are to take on future roles involving pharmacogenomics. Surveys conducted in the United States have discovered that, although some instruction in pharmacogenomics is currently provided, it needs to be enhanced and developed to keep current.17,18
Since pharmacists are well prepared to analyze drug issues, and are accessible to provide information to patients, they should have formal pharmacogenomics training to maximize their impact in health care.
MEDICATION THERAPY MANAGEMENT
Pharmacists are trained to be the medication expert on the health care team. Experts estimate that 1.5 million preventable adverse events occur each year, resulting in $177 billion in injury and death from medication-related problems and medication mismanagement.19 Pharmacogenomics can be a useful tool for pharmacists to use in preventing medication-related problems and adverse events with drugs such as abacavir and warfarin. Medication therapy management is a term used to describe a broad range of health care services provided by pharmacists and other members of the health care team. As defined in a consensus statement adopted by the pharmacy profession in 2004, medication therapy management is “a service or group of services that optimize therapeutic outcomes for individual patients.”20 Medication therapy management services include, but are not limited to, medication therapy reviews, pharmacotherapy consults, anticoagulation management, immunizations, and health and wellness programs. Integration of pharmacogenomic information into these services is a logical extension of current practice. Pharmacists are the most likely professional to offer these services, but specially trained or advanced practice nurses may also provide them. Medication therapy management optimizes the benefits patients obtain from their medications by actively managing drug therapy and identifying, preventing, and resolving medication-related problems. One example of integration of pharmacogenomic data into medication therapy management would be utilizing CYP2C19 to identify patients who will not respond to clopidogrel therapy.
Medication therapy management services are offered in all care settings in which patients take medications. The goal of medication therapy management, no matter the setting, is to ensure that each medication is right for the patient and his or her health conditions and that the best possible outcomes from treatment are achieved. Integration of pharmacogenomics into the existing rubric for medication therapy management services allows the tailoring of medication therapy more precisely for the patient.
PATIENT-CENTERED MEDICAL HOME
The patient-centered medical home is an approach to providing comprehensive primary care to adults, youth, and children. It may broaden access to primary care while enhancing care coordination. Providing care to patients in this type of approach encourages clinicians to practice in a collaborative effort. This collaboration is particularly important because pharmacogenomic data do not currently have a standardized place in electronic medical records (EMRs) or pharmacy dispensing systems.
Clinicians can educate patients on preventative care utilizing environmental and genetic risk factors in the patient-centered medical home model. Working together, clinicians initiate treatment and prevention measures before costly, last-minute emergency procedures are required. Utilizing HLA-B*5701 to prevent abacavir-associated hypersensitivity reaction (HSR) is one example of integrating pharmacogenomic data to prevent patient harm. The role of the patient-centered medical home in coordinating care should result in healthier patients and using pharmacogenomic data should result in safer and more effective medication use. The consideration and evaluation of pharmacogenomic tests in this setting emphasizes the need for pharmacogenomic education for all health professionals.
Integration of Pharmacogenomics into Medication Prescribing Information or Package Inserts
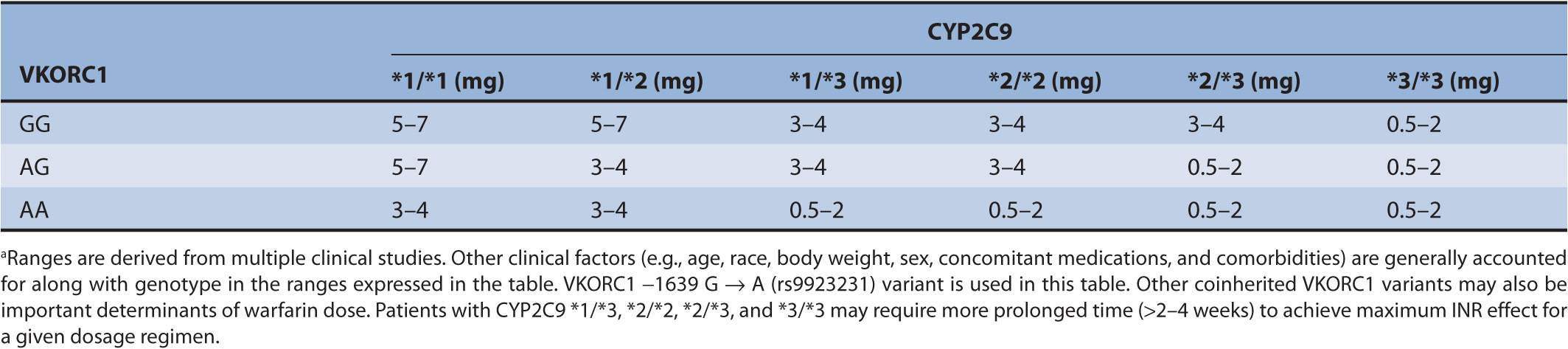
When health care professionals have a question regarding the relationship between a genomic biomarker and a medication, most refer to the drug prescribing information in the package insert. In 2006, a study performed by Frueh et al. reviewed the approved drug labels in the United States to find that 121 out of approximately 1,200 drug labels contained genomic information.21 Of the 121, only 69 contained information about normal or cancerous human genetic information. The remaining 52 labels contained viral or microbial genomic information. While the US Food and Drug Administration requires the inclusion of human genomic information in a number of drug package inserts, testing prior to prescribing is not mandated.22 As an example, the drug label for warfarin provides a genotype-based dosing using cytochrome P 450 2C9 (CYP2C9) and the vitamin K epoxide reductase subunit 1 (VKORC1) as data that may guide prescribing to achieve a therapeutic international normalized ratio (INR). (The INR is a lab value monitored to help ensure safe and effective drug therapy with warfarin.) However, the package insert does not require the prescriber to obtain, but rather describes the use of, the pharmacogenomic information “when it is available”23 (see Table 9–1).
TABLE 9–1 Warfarin dosing based on genotype (from warfarin package insert).a
This lack of a provision for pharmacogenomics testing prior to dosing can be seen in at least 69 other package inserts. As more data demonstrate the value of pharmacogenomic testing prior to prescribing drugs, patients or prescribers will begin to request pharmacogenomic testing to personalize drug therapy, and pharmacists and other health care professionals will find prescribing guidance in the package inserts or on the US FDA Web site.22
PUBLIC ACCESS TO TESTING: TYPES OF TESTS
Direct-to-Consumer Pharmacogenomic Tests
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree