http://evolve.elsevier.com/McCuistion/pharmacology/
Since the acquired immunodeficiency syndrome (AIDS) epidemic began in the United States in the early 1980s, advancement of antiretroviral therapy (ART) has dramatically improved human immunodeficiency virus (HIV)-related morbidity and mortality and has reduced perinatal and behaviorally associated HIV transmission. The Centers for Disease Control and Prevention (CDC) reports that more than 1.2 million people in the United States are infected with HIV; between 10% and 15% of persons living with HIV do not know they have it. Approximately 50,000 new cases of HIV infection occur annually. Improvement of health, prolonging lives, reducing transmission risk, and suppression of HIV necessitates the use of combination treatments with antiretroviral drugs. Current clinical guidelines recommend ART for all persons infected with HIV-1, regardless of their viral load (CD4+ cell count). Prophylactic treatment of HIV-negative partners minimizes their risk of contracting HIV. It is critical that nurses educate patients regarding ART and, in partnership with the patient, develop strategies to optimize adherence.
Although strides have been made in the treatment of HIV/AIDS, challenges remain. One important aspect is increased drug resistance to current therapies. The makeup of the HIV DNA strands allows the virus to mutate from a drug-sensitive to a drug-resistant form. To minimize resistant strains, clinical and nonclinical providers and health departments need to strive for the highest possible adherence to ART. At each health care visit, persons with HIV and their partners should receive ongoing counseling to reinforce HIV prevention measures, screening for high-risk behaviors, diagnosis and treatment of sexually transmitted infections (STIs), and reinforcement of the importance of medication adherence. Open communication between the health care provider and patient should be fostered.
The goals for initiating ART are to (1) reduce HIV-associated morbidity and mortality, (2) prolong the duration and quality of life, (3) restore and preserve immunologic function, (4) maximally and durably suppress plasma HIV viral load, and (5) prevent HIV transmission.
HIV Infection: Pathophysiology
HIV is an RNA retrovirus. It is unable to survive and replicate unless it is inside a living human cell. HIV destroys CD4+ T cells, also called helper T cells or CD4+ T lymphocytes; these play a critical role in the human immune response through recognition of infectious and neoplastic processes. The destruction of CD4+ cells by HIV results in immune deficiency, so the CD4+ cell count is an indicator for immune function in those with HIV.
Normal CD4+ counts range from 500 to 1200 cells/mm3. After initial infection, rapid viral replication occurs, resulting in a high level of HIV in circulation (high viral load); the virus then attacks and destroys CD4+ cells. There is a corresponding drop in CD4+ cells, which triggers an immune response that results in CD4+ cell replacement and HIV antibody production. HIV uses the CD4+ cell’s apparatus to replicate itself and spread throughout the body. The CD4+ cells continue to drop as HIV viral load increases, which further weakens the person’s natural immune system such that it cannot fight off infection and disease (e.g., cancer). Symptoms of HIV infection range from mild to severe and include fever, fatigue, pharyngitis, myalgia or arthralgia, lymphadenopathy, headache, and night sweats in those recently infected; these symptoms can be experienced 2 to 12 weeks after HIV exposure. This period is called acute retroviral syndrome, acute seroconversion syndrome, or primary HIV infection. At this stage, people are highly infectious and symptoms can often be mistaken, by both patient and health care provider, for a transient flulike illness. Consequently, few people are diagnosed during this time. Additionally, the time delay from infection to a positive HIV test result—the so-called window period—averages 10 to 14 days, but some do not seroconvert for 3 to 4 weeks. Almost all patients seroconvert within 6 months. For this reason, patients who are at risk for HIV infection and test negative should be counseled to have the test repeated in 3 months (the close of the window period). If HIV is strongly suspected, an HIV RNA quantitative test can be done. A viral load of 10,000 copies/mL usually indicates the virus is adequately suppressed.
HIV Life Cycle
The phases of the HIV life cycle (Fig. 29.1) are binding, fusion, reverse transcription, integration, replication, assembly, and budding. HIV begins its life cycle when it binds to CD4+ receptors on the surface of a CD4+ T lymphocyte. The virus then fuses with the host cell, allowing entrance into the CD4+ cell. After fusion, the virus releases RNA, its genetic material, into the host cell to be converted into HIV DNA. This reverse transcription (RT) lets the virus enter into the host cell’s nucleus and integrate (combine) with the cell’s DNA. The integrated HIV DNA, a provirus, creates copies of the HIV genomic material (replication) and shorter strands of RNA called messenger RNA (mRNA). The mRNA is used as a blueprint to make long chains of HIV proteins. The new HIV proteins and HIV RNA assemble into an immature, noninfectious HIV, which then pushes out of the host cell (budding). Finally, these immature HIV cells release HIV enzymes (proteases) that disassemble the long protein chains of immature HIV virus. The now individual proteins can combine to make copies of mature HIV and can then move on to infect other cells.
HIV Transmission
HIV is spread via intimate contact with blood, semen, vaginal fluids, and breast milk. Transmission of the virus occurs primarily by (1) sexual contact, which includes oral, vaginal, and anal sex; (2) direct blood contact including intravenous (IV) drug use with shared needles or shared drug works, shared contaminated personal care items such as razors, and blood transfusions (now extremely rare in the United States); and (3) mother-to-child contact through shared maternal-fetal blood circulation, direct blood contact during delivery, or breast milk. Included in these modes are accidental needle injury, artificial insemination with donated semen, and organ transplant. Those at highest risk include persons who engage in unprotected sex, those with multiple sexual partners (either the patient or partner[s] of the patient), IV drug users who share needles or drug works, and infants born to women with HIV. The risk of mother-to-child transmission (MTCT) is 25% without ART; the risk decreases to 1% to 2% with successful use of ART. Other factors that increase the risk of MTCT are a mother with a viral load greater than 1000 copies/mL at delivery, premature rupture of the membranes, hepatitis C virus coinfection, preterm gestation, and vaginal delivery. HIV is not spread by air or water, mosquitoes or ticks, shaking hands, hugging, sharing toilets, sharing dishes or drinking glasses, or drinking fountains.
Laboratory Testing
Several laboratory tests are important for initial patient evaluation upon entry to care, during follow-up evaluation for those not on ART, and before and after initiation or modification of ART to assess for immunologic and virologic efficacy of treatment.
The laboratory tests used to determine when to initiate medication therapy and to monitor efficacy of therapy and indications for changing therapy are CD4+ T-cell count, plasma HIV RNA quantitative assay (or viral load test), and HIV resistance testing.
The count reflects the number of CD4+ cells circulating in the blood. The result is listed as an absolute number and a relative percentage. The absolute count can vary in the same patient depending on the laboratory used, the time of day laboratory blood work is drawn, or acute illness. The CD4+ percentage is a more stable reflection of the immune system and is used in conjunction with the absolute count to monitor health status and response to medication therapy. The patient should be encouraged to use the same laboratory at approximately the same time of day to promote consistency of results. The nurse should monitor the laboratory used because lab value references vary from laboratory to laboratory. The HIV viral load is indicative of the level of virus circulating in the blood and is the best determinant of treatment efficacy. A key goal of therapy is to achieve and maintain a viral load below the limits of detection (<20 to 40 copies/mL, depending on the assay used). This goal should be achieved in 16 to 24 weeks of therapy. Resistance to ART leads to treatment failure and the risk of transmitting drug-resistant virus. Determination of the presence of a drug-resistant strain of HIV is important to prevent ineffective treatment. Individuals who experience failure of ART should have drug-resistance testing, assessment of drug adherence, and a review of possible drug-drug and drug-food interactions and drug intolerability, treatment history, and HIV RNA and CD4+ cell counts.
Classification
HIV disease staging and classification systems are important tools for tracking and monitoring the HIV epidemic and for providing the clinician and patient with information about HIV disease stage and clinical management. The two major classification systems are the CDC staging system (revised in 2014) and the World Health Organization (WHO) system (revised in 2015).
The CDC system assesses the severity of HIV disease by CD4+ cell counts and by presence of specific HIV-related conditions; the system is based on the lowest documented CD4+ cell count (nadir CD4+) and on previously diagnosed HIV-related conditions. The WHO system is useful in resource-constrained settings without access to CD4+ cell measurements and classifies HIV disease based on clinical manifestations that clinicians and those with varying levels of HIV expertise and training can recognize in diverse settings.
Indications for Antiretroviral Therapy
ART has dramatically reduced HIV-associated morbidity and mortality. However, the CDC reported that in the United States, fewer than one-third of HIV-infected individuals have adequate viral suppression. Many individuals with HIV infection are undiagnosed or they are not retained within the health care system for routine monitoring.
A set of guidelines was developed by the U.S. Department of Health and Human Services Expert Panel on Antiretroviral Guidelines for Adults and Adolescents (the Panel). The Panel last updated the guidelines in 2016 and recommends ART for all HIV-infected individuals regardless of CD4+ count to reduce HIV-related morbidity and mortality and to prevent HIV transmission. The Panel also seeks to educate patients on the benefits and considerations for ART, especially the importance of adherence. The current guidelines for treatment-naïve persons with HIV include initial therapy with two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a third active antiretroviral (ARV) drug from one of three drug classes: an integrase strand transfer inhibitor (INSTI), a nonnucleoside reverse transcriptase inhibitor (NNRTI), or a protease inhibitor (PI) with a pharmacokinetic (PK) enhancer (booster) (cobicistat or ritonavir). All HIV-infected persons diagnosed with active tuberculosis (TB) should be started on antiretroviral and TB therapy. Rifamycin (rifabutin, rifampin) should be included in the TB regimen despite drug interactions. Chapter 27 further discusses antituberculars.
Suppression of HIV with ART may decrease inflammation and immune activation that is thought to contribute to higher rates of cardiovascular, kidney, and liver disease; neurologic complications; and malignancy in HIV-infected cohorts. If therapy is to be initiated, medications are selected based on results of genotypic resistance testing where applicable; comorbidities (e.g., liver disease, renal dysfunction, depression); potential drug-drug interactions; pregnancy status; and assessment of the patient’s willingness and readiness to start therapy. Evaluation of medication readiness should include dosage regimen, pill burden, dosing frequency, food restrictions, side effects, and the patient’s daily routine. Tools for promoting medication adherence (e.g., alarms, pill planners) and plans for management of potential medication side effects should be reviewed before medication initiation. The patient should be instructed in the need for a better than 95% medication adherence, the potential for development of medication resistance with less than optimal adherence, and the clinical implications of resistance.
Antiretroviral Drugs
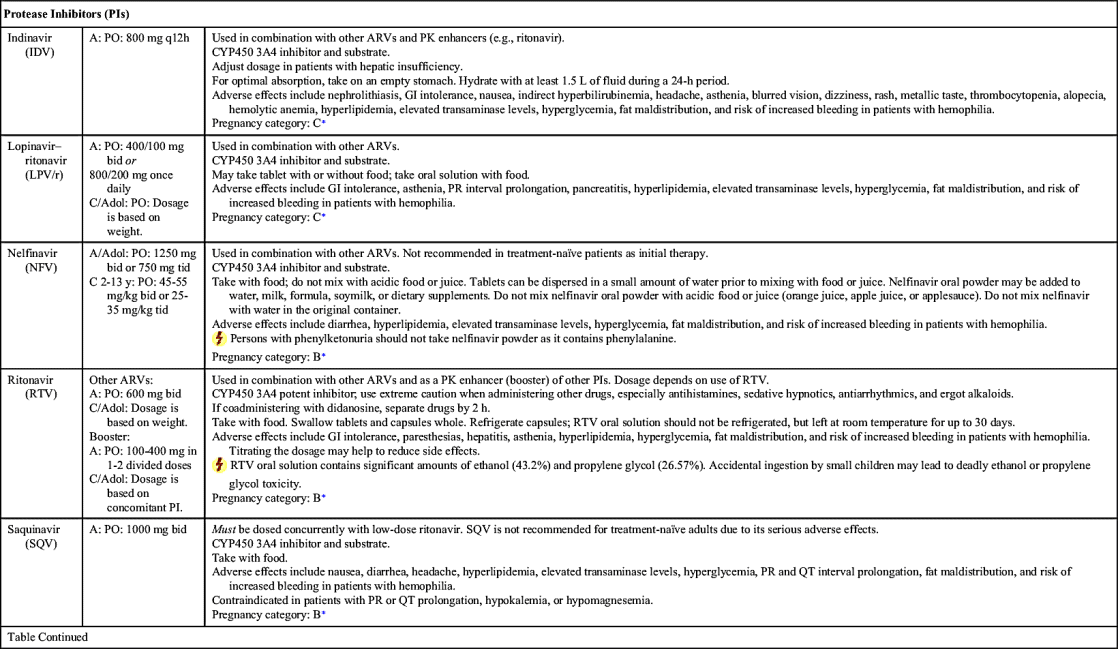
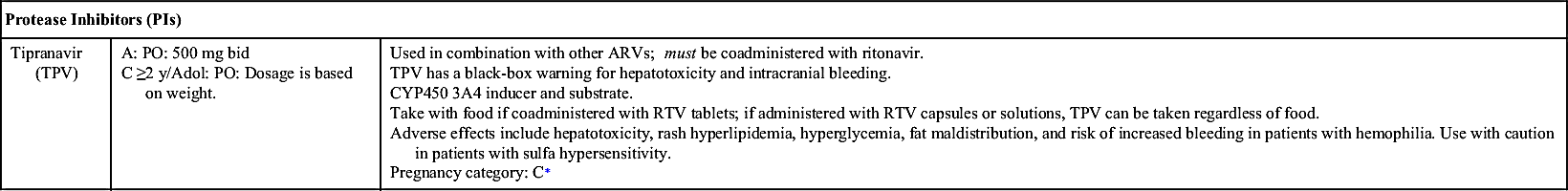
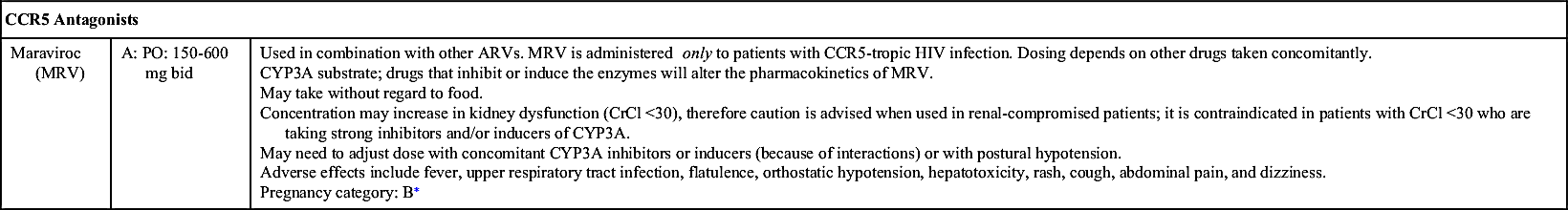
NRTIs, NNRTIs, PIs, fusion (entry) inhibitors, CCR5 antagonists, and INSTIs make up the drugs used as ART. Table 29.1 lists dosages, uses, and considerations for several ARVs. More than 25 different ARVs have received U.S. Food and Drug Administration (FDA) approval, and various agents are available in fixed-dose combinations that contain two or more HIV medications from one or more drug classes. In addition to the above classes of antiretrovirals, PK enhancers are approved to be taken with some ARVs (e.g., PIs and certain INSTIs).
Since the 1980s, when zidovudine monotherapy showed survival benefits in advanced HIV patients, much progress has been made. Newer agents have improved adherence (e.g., fewer pills for more convenient dosing, formulation changes that reduce dosing frequency or pill burden, combination dosage forms with two or three drugs in one pill). Other improvements include increased potency, improved side-effect profile (e.g., decreased gastrointestinal [GI] effects), and PI enhancers.
ART is the standard of care in the treatment of HIV infection. Currently, there are six recommended regimens for treatment-naïve patients, five of which are INSTI based and one that is ritonavir-boosted–PI based. For treatment-naïve patients, ART generally consists of two NRTIs plus an INSTI, NNRTI, or PK-enhanced PI. Treatment-experienced patients who encounter drug resistance should begin a new regimen that includes two to three fully active drugs. Adding a single ARV is not recommended because of the increased risk of drug resistance to all ARVs. Furthermore, drug interruption is not recommended due to a risk of rapid increase of HIV RNA viral load and a decrease in CD4+ cell count.
The Panel reports that nevirapine (NVP) should not be given to ARV-naïve women whose CD4+ count is greater than 250 cells/mm3 or to men with CD4+ greater than 400 cells/mm3 due to a high incidence of hepatotoxicity; however, if no other ARV option is available, NVP can be administered, but patients should be closely monitored. On the other hand, the Panel reports no exceptions to stavudine plus zidovudine (antagonistic effect) and unboosted darunavir, saquinavir, or tipranavir (inadequate bioavailability).
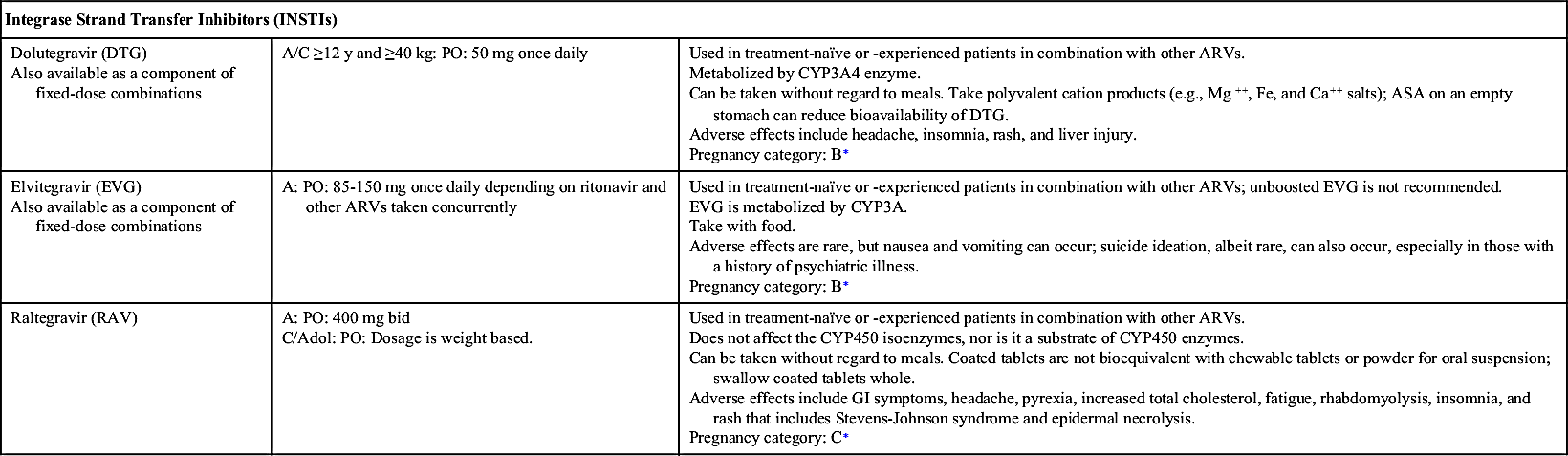
INSTI-based regimens include the following:
• Dolutegravir-abacavir-lamivudine (DTG-ABC-3TC), but only for human leukocyte antigen (HLA)-B∗5701–negative patients (presence of the HLA-B∗5701 allele increases the risk of hypersensitivity to abacavir and is not recommended to individuals positive for the gene)
• Dolutegravir plus tenofovir disoproxil fumarate–emtricitabine (DTG plus TDF-FTC)
• Elvitegravir–cobicistat–tenofovir alafenamide–emtricitabine (EVG-c-TAF-FTC), but only for patients with pretreatment creatinine clearance (CrCl) greater than or equal to 30 mL/min
• Elvitegravir–cobicistat–tenofovir disoproxil fumarate–emtricitabine (EVG/c/TDF/FTC), but only for patients with pretreatment CrCl greater than or equal to 70 mL/min
• Raltegravir plus tenofovir disoproxil fumarate–emtricitabine (RAL plus TDF/FTC)
PI-based regimens include ritonavir-boosted darunavir plus tenofovir disoproxil fumarate–emtricitabine (DRV/r plus TDF/FTC).
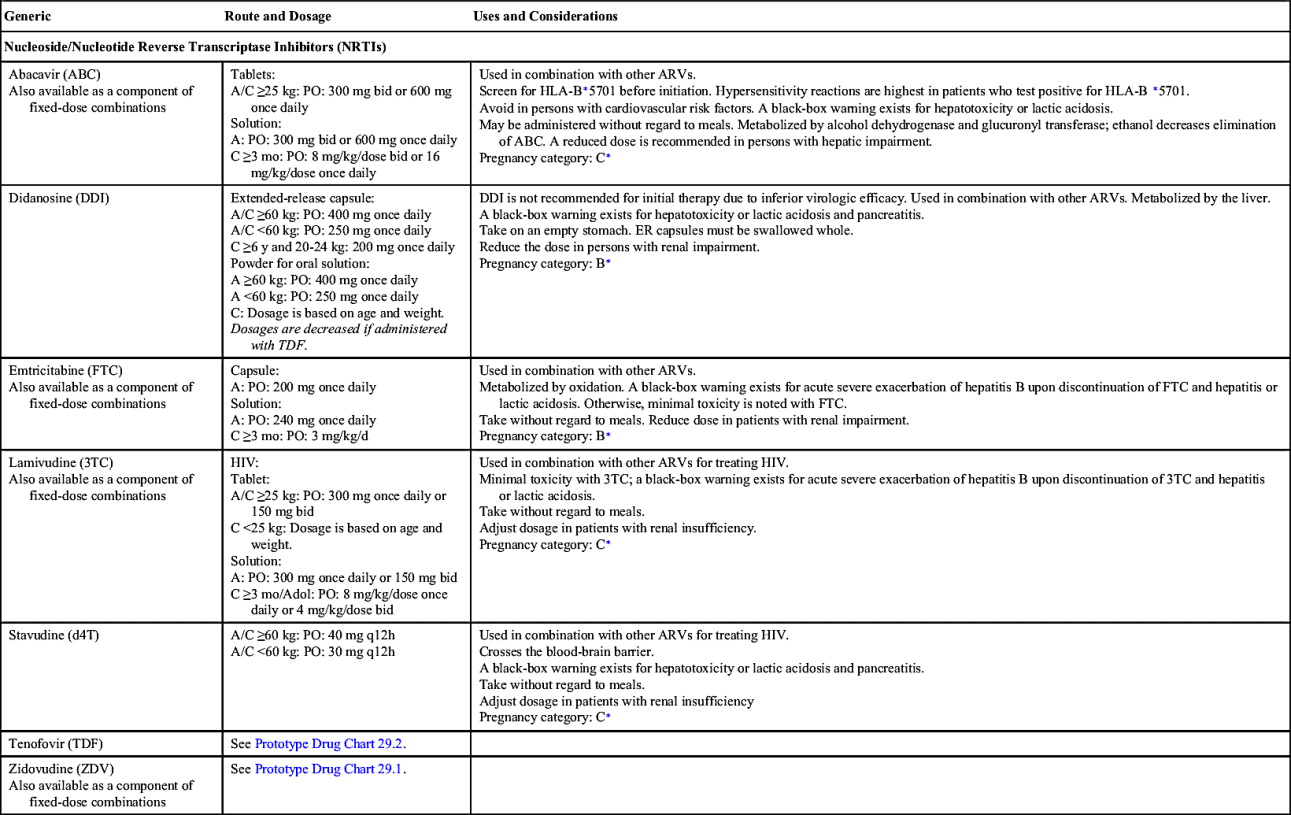
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
Of the antiretroviral drugs, NRTIs were the first type of drug to treat HIV. NRTIs act by interfering with HIV viral RNA-dependent DNA polymerase, resulting in inhibition of viral replication. Seven NRTIs, also known as “nukes,” are approved for use in the United States: zidovudine, didanosine, stavudine, lamivudine, abacavir, tenofovir, and emtricitabine. All but didanosine and stavudine are available in a fixed-dose combination with other classes of ARV drugs.
All NRTIs except didanosine can be taken without regard to food. Didanosine should be taken 30 minutes before or 2 hours after meals for optimal absorption. Fifty percent or more of NRTIs are excreted by the kidneys, therefore NRTIs require dosage adjustment in persons with renal insufficiency. With abacavir, dosage adjustment is recommended in individuals with hepatic insufficiency.
As a class, NRTIs are associated with changes in the body’s metabolism secondary to mitochondrial toxicity. GI side effects such as nausea, diarrhea, and abdominal pain are transient and improve within the first 2 weeks of therapy. Rash is a common hypersensitivity reaction. Complications include peripheral neuropathy, myopathy, pancreatitis, and lipoatrophy. Lipoatrophy—or wasting of fat on the extremities, face, and buttocks—is associated with chronic NRTI administration. Rare fatalities have occurred due to lactic acidosis and hepatic steatosis associated with NRTIs. Persons coinfected with hepatitis B virus (HBV) are at risk for severe acute exacerbation of their HBV upon discontinuation of emtricitabine, lamivudine, or tenofovir disoproxil fumarate.
Drug interactions are minimal with NRTIs because these drugs are not metabolized by the cytochrome P450 (CYP450) isoenzymes. However, drug interactions can still occur: for example, ribavirin inhibiting phosphorylation of zidovudine can cause hematologic toxicities; and coadministration of ribavirin with didanosine is contraindicated because fatal hepatic failure can occur; didanosine coadministered with stavudine can worsen lactic acidosis and pancreatitis; allopurinol is contraindicated in individuals taking didanosine; and PIs can increase serum concentration of tenofovir disoproxil fumarate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree