Hereditary Cancer
HEREDITARY BREAST CANCER
Familial Breast Cancer
Long recognized that many women with breast cancer also have affected relatives
˜ 10-25% of patients have a 1st-degree relative (parent, sister, daughter) with breast cancer
Having affected 1st-degree relative increases patient risk by 2-3x
Women whose only family history is mother developing postmenopausal cancer are not at increased risk
Majority of postmenopausal breast cancers are sporadic
5-10% of breast cancers have been linked to specific high penetrance germline mutations
High penetrance = RR > 10
Moderate penetrance = RR 2-3
Low penetrance = RR < 1.5
Penetrance may be gender dependent for associated cancers (e.g., female carriers are at higher risk for breast cancer than males)
Remainder of familial risk is likely due to multiple genes of lower penetrance
Unlikely that another highly penetrant gene comparable to BRCA1 or 2 will be identified
Most Common Germline Mutations
Majority of germline mutations associated with breast cancer risk are involved in DNA repair pathways
Tissue specificity for breast cancers is not understood
Mutations are autosomal dominant alleles and thus can be inherited via both females and males
BRCA1 (familial breast and ovarian cancer)
BRCA1 and 2 help maintain genomic stability
Direct role in regulation of DNA-damage responses and repair and cell cycle checkpoints
Inactivating mutations impair conservative DNA repair and genomic stability functions
Cells lacking BRCA1 & 2 functional activity prone to replication errors and genomic instability
Accumulating DNA abnormalities enable mutations in genes essential to cell cycle check point activation
Drives acquisition of mutations and chromosomal instability, contributing to tumor formation
BRCA1 function is required for transactivation of the estrogen receptor gene promoter
May explain why 90% of BRCA1-associated carcinomas are ER(-)
Incidence: ˜ 1 in 860
More common in some ethnic groups: Ashkenazi Jews, Finns, French Canadians
High penetrance: 45-70% lifetime risk
Magnitude of risk can vary for different mutations and for different types of cancers
Responsible for ˜ 1/2 of cancers known to be due to a germline mutation (˜ 2% of all breast cancers)
Other associated cancers
Ovarian (40-50% lifetime risk), fallopian tube, peritoneal, and pancreatic cancer
Male breast cancer (1-5% lifetime risk, but ˜ 1/2 risk of BRCA2)
˜ 90% of BRCA1 cancers share same gene expression pattern with basal-type carcinomas
Basal carcinomas may also have defective BRCA1 function
Cancers share morphologic features
Mutation can be suspected in young patients (35% risk if grade 3 cancer is ER(-) and patient < 30 years of age)
BRCA2 (familial breast and ovarian cancer syndrome)
Although BRCA2 is not structurally related to BRCA1, the functions of these genes are very similar
Incidence: ˜ 1 in 740
More common in some ethnic groups: Ashkenazi Jews, Icelandic populations
High penetrance: 40-60% lifetime risk
Magnitude of risk can vary for different mutations and for different types of cancers
Responsible for ˜ 1/3 of cancers known to be due to a germline mutation (˜ 1% of all breast cancers)
Other associated cancers
Ovarian (10-20% lifetime risk), fallopian tube, prostate, pancreatic, gallbladder, stomach, bile duct cancers, melanoma
Male breast cancer: 5-10% lifetime risk; approximately 5-15% of cases are associated with BRCA2
Cancers group with luminal A type by gene expression profiling
CDH1 (familial gastric cancer and lobular breast cancer syndrome)
CDH1 encodes the gene for E-cadherin
Inherited mutations increase risk for signet ring cell carcinomas of stomach and lobular carcinoma
Majority of women with lobular carcinomas do not have CDH1 germline mutations
40-85% lifetime risk of developing gastric signet ring cell carcinoma in majority of families
Gastric carcinomas are more common than breast carcinomas in most affected families
Families developing only breast cancer have also been identified
PTEN (Cowden syndrome)
Dual specificity phosphatase gene involved in apoptosis
Incidence: ˜ 1 in 300,000
Characterized by multiple hamartomas (including trichilemmomas)
Breast lesions include fibroadenomas and hamartomas
Increased risk of breast, thyroid, and endometrial cancer
Women have a 25-50% lifetime risk of breast cancer
Men also at increased risk for breast cancer
TP53 (Li Fraumeni syndrome)
P53 has central role in cell cycle control, DNA replication, DNA repair, and apoptosis
Increased risk of soft tissue sarcoma, osteosarcoma, breast cancer, brain tumors, leukemia, adrenocortical cancer (90% of patients develop some type of tumor by age 70)
Breast cancer is 1/3 of malignancies in affected families; found in ˜ 1% of women with breast cancer < age of 40
˜ 55% of women will develop breast cancer by age 45 (average age at diagnosis is 36)
Other tumors, particularly sarcomas and brain tumors, can develop in childhood
30% of tumors occur in individuals 15 years of age or younger
Somatic P53 mutations are also frequently found in BRCA1 and 2 associated carcinomas
ATM (ataxia-telangiectasia carriers)
Serine threonine kinase that phosphorylates TP53 and BRCA1 in response to DNA double strand breaks
Incidence: 0.2-1%
Homozygosity results in ataxia-telangiectasia
Progressive cerebellar ataxia, oculocutaneous telangiectasias, immunodeficiency, and increased risk of leukemia and lymphoma
Heterozygosity increases risk of breast cancer
11% by age 50 and 30% by age 70
CHEK2 (Li Fraumeni variant syndrome)
Cell cycle checkpoint gene involved in DNA repair
Incidence: 1 in 100
Low penetrance: 10-20% lifetime risk of breast cancer for females and males
STK11/LKB1 (Peutz-Jeghers syndrome)
Serine/threonine kinase that functions as a tumor suppressor
Incidence: 1 in 20,000
Characterized by hamartomatous gastrointestinal polyps (including small intestine) and skin pigmentation (lips and buccal mucosa)
Increased risk of cancers of colon, breast, stomach, pancreas, small intestine, thyroid, lung, uterus, ovaries, cervix
BRIP1 (FANCJ or BACH1)
DNA helicase that interacts with BRCA1 to carry out its functions
Mutations result in defects in DNA repair
Homozygosity results in Fanconi anemia
Heterozygosity increases risk of breast cancer
Low to moderate penetrance
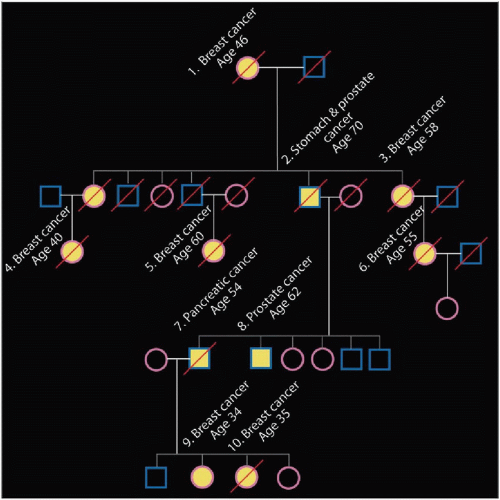
Patterns of Familial Cancer Likely to be Associated with Germline Mutations
Affected 1st-degree relatives (mother, sister, daughter)
Multiple relatives affected, particularly if 1st degree
Carcinomas occurring at an early age (premenopausal)
Individuals with history of multiple cancers
Relatives with non-breast cancers associated with particular germline mutation
Male breast cancer for BRCA2 or 1
Ovarian cancers for BRCA1 and 2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree