Figure 17–1 Iron metabolism. Ingested iron is absorbed from the intestinal mucosa into the circulation, where it is bound to transferrin. Iron is distributed to tissues for incorporation into hemoglobin, myoglobin, and enzymes, or it is stored as ferritin. After about 120 days, erythrocytes are degraded by reticuloendothelial cells, and the iron is returned to the plasma or stored.
The dietary iron requirement per kilogram of body weight is highest in infants and pregnant women, somewhat lower in children and nonpregnant women, and lowest in men. Pregnant women have the greatest need for routine iron supplementation because their dietary iron often cannot meet the requirements for maternal and fetal erythropoiesis. Most multivitamin supplements contain iron, and many processed foods, including bread, are supplemented with iron.
If dietary iron intake is inadequate to support sufficient erythropoiesis and maintain a normal hemoglobin concentration in the blood, the body will utilize stored iron to maintain erythropoiesis until the stores are depleted. When iron stores are significantly depleted, the plasma iron level begins to fall and erythropoiesis is reduced. Over time, these changes lead to hypochromic microcytic anemia, a form of anemia in which the mean corpuscular hemoglobin concentration and the mean corpuscular volume are decreased. The iron preparations described in the following paragraphs are used to prevent and treat iron deficiency anemia in affected individuals.
Ferrous Sulfate and Related Compounds
Iron is administered orally in the form of ferrous salts, including ferrous sulfate, ferrous gluconate, and ferrous fumarate. Iron contained in these preparations is absorbed in the same manner as is dietary iron. In patients with iron deficiency, the amount of iron absorbed increases progressively with larger doses, but the percentage absorbed decreases as the dosage increases. Food can retard iron absorption by 40% to 60%, but the gastric distress caused by iron often necessitates administering iron preparations with food. The percentage of iron absorbed from sustained-release preparations is lower than that absorbed from immediate-release preparations. This is because iron is primarily absorbed from the duodenum, and some of the sustained-release iron is transported to the lower intestinal tract before it is released for absorption.
In iron deficiency states, oral iron preparations are usually administered three times a day in doses that provide a total of 100 to 200 mg of elemental iron daily. The various iron salts contain different percentages of elemental iron. Ferrous sulfate contains about 20%, so that a 300-mg tablet contains approximately 60 mg of elemental iron. The duration of iron therapy depends on the cause and severity of the iron deficiency. In general, about 4 to 6 months of oral iron therapy is required to reverse uncomplicated iron deficiency anemia.
At therapeutic doses, iron salts have few adverse effects, but they sometimes cause epigastric pain, nausea, vomiting, diarrhea or constipation, and black stools. Liquid iron preparations can also stain the teeth. Bile acid–binding resins (e.g., cholestyramine) reduce the absorption of iron, whereas ascorbic acid increases iron absorption by maintaining iron in the reduced ferrous state. Ferrous iron is better absorbed than is ferric iron. Iron can reduce the absorption of tetracyclines, fluoroquinolones, levothyroxine, and vitamin E. The administration of these drugs should be separated from iron administration by at least 2 hours. The ingestion of large quantities of iron can cause serious and potentially lethal toxicity. Hence, iron preparations should be kept out of the reach of children.
Iron Dextran
Iron dextran is a mixture of ferric hydroxide and dextran. It is intended for intramuscular or intravenous treatment of iron deficiency anemia in patients who cannot tolerate oral iron preparations or fail to respond to oral iron therapy. The dosage of iron dextran required for each patient is calculated on the basis of the observed hemoglobin concentration and body weight.
Following administration, the iron dextran complex is removed from the circulation by the reticuloendothelial system, and the iron is transferred to the plasma for distribution to the bone marrow and other tissues.
Because treatment with iron dextran has been associated with fatal anaphylactic reactions, it should be limited to the indications described. Intramuscular administration of iron dextran can cause several adverse reactions at the injection site, including pain, inflammation, sterile abscesses, and brown discoloration of skin. For this reason, the iron preparation must be given by deep intramuscular injection into the outer quadrant of the buttock. A Z-track technique, in which the skin is displaced laterally before injection, is used to avoid leakage into the subcutaneous tissue. Intravenous administration can cause peripheral flushing and hypotensive reactions.
Vitamins
Although many vitamins participate in the formation and function of erythrocytes, folic acid and vitamin B12 have a critical role in erythropoiesis, and a deficiency of either of them may cause megaloblastic anemia. The two vitamins serve as cofactors in biochemical reactions involving the addition of single-carbon units to various substrates, and the administration of one of the vitamins can partially compensate for a deficiency of the other. Therefore, it is critical that the specific vitamin deficiency be correctly identified before therapy is started.
Folic Acid
The structure of folic acid is shown in Figure 17–2A. Active forms of this vitamin serve as enzyme cofactors that donate single-carbon atoms in the biosynthesis of amino acids and the purine and pyrimidine bases contained in DNA (Fig. 17–3A). Hence, folic acid plays a critical role in cell proliferation and erythropoiesis.
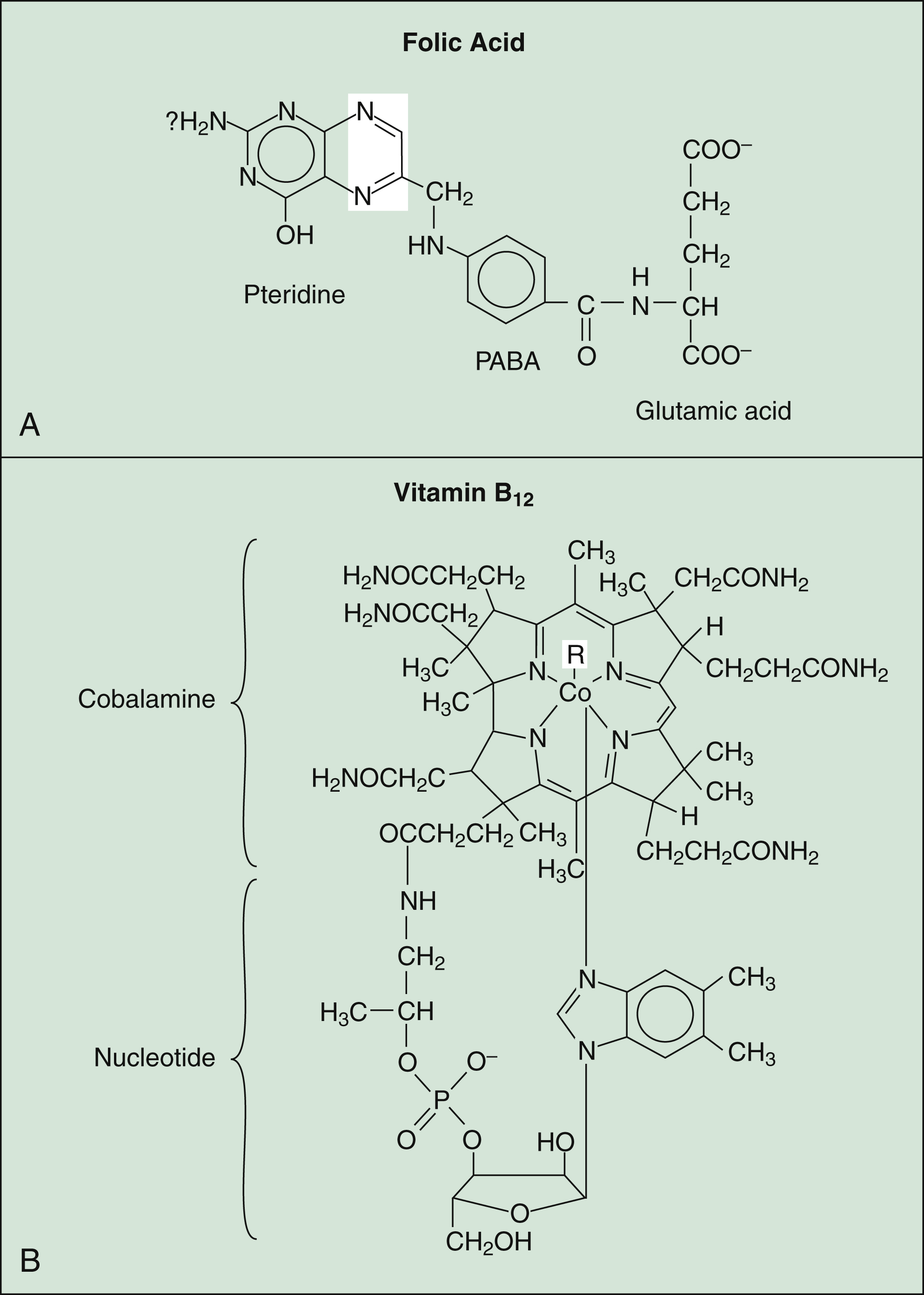
Figure 17–2 Structures of folic acid and vitamin B12. (A) Folic acid consists of pteridine, PABA, and glutamic acid. The unshaded areas represent the bonds that are reduced by folate reductase to form tetrahydrofolic acid. (B) Vitamin B12 consists of a porphyrin-like ring with a central cobalt (Co) atom attached to a nucleotide. PABA = p-aminobenzoic acid; R = CN− (cyanocobalamin) or OH− (hydroxocobalamin).
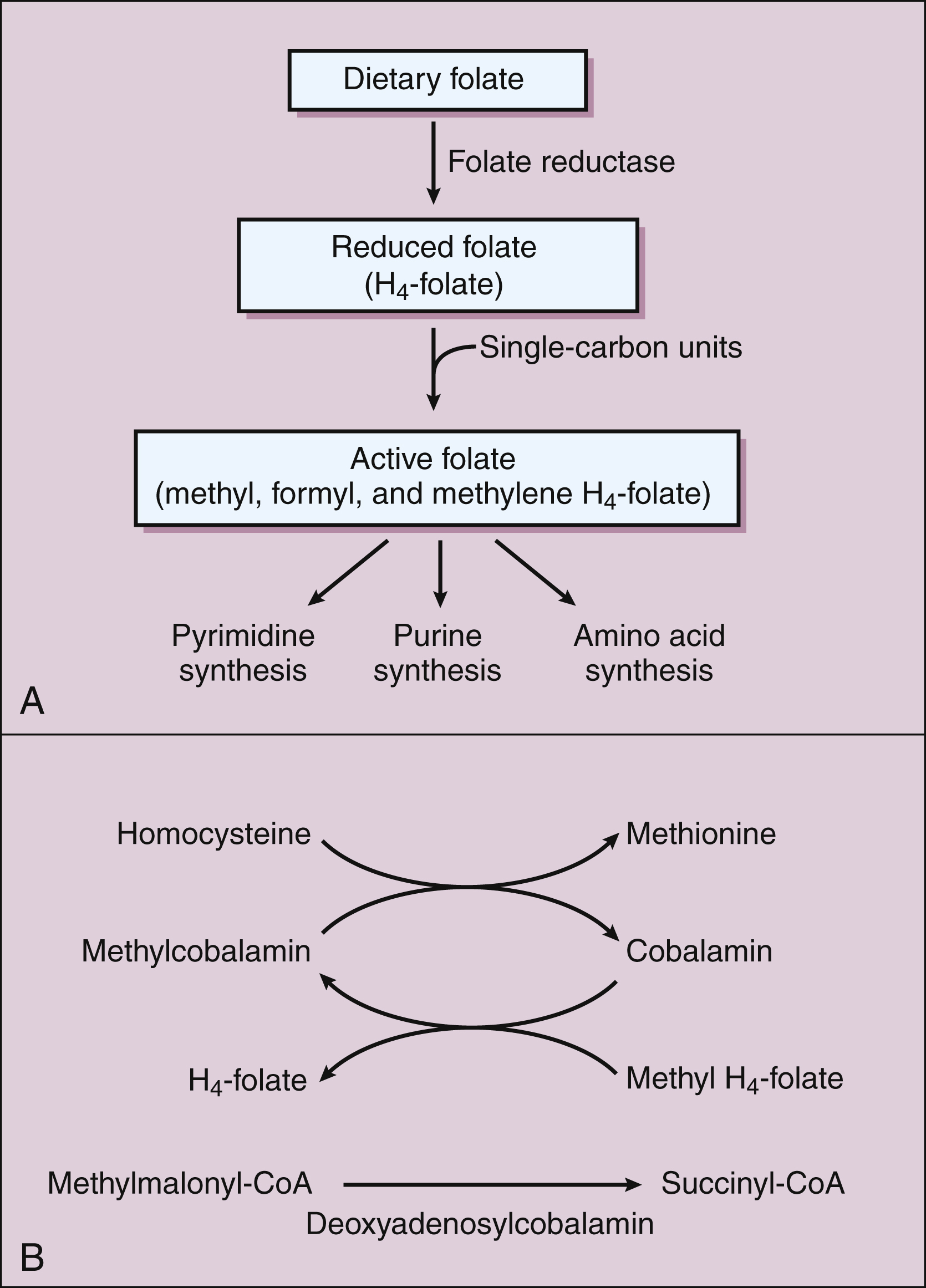
Figure 17–3 Biochemical reactions involving folic acid and vitamin B12. (A) Dietary folate is reduced by folate reductase to tetrahydrofolate (H4-folate). Single-carbon units are added to H4-folate to form active folate, which donates single-carbon units in the synthesis of pyrimidine and purine bases and amino acids. (B) The two active forms of vitamin B12 are methylcobalamin and deoxyadenosylcobalamin. These forms are cofactors for methylation reactions, including the conversion of homocysteine to methionine and the conversion of methylmalonyl-CoA to succinyl-CoA. Methyl H4-folate donates a methyl group to cobalamin to form methylcobalamin.
The requirement for folic acid increases markedly during pregnancy, and inadequate dietary intake of this vitamin can cause neural tube birth defects, such as spina bifida, and megaloblastic anemia. For this reason, folic acid supplementation is especially important before and during pregnancy. The standard U.S. diet provides 50 to 500 μg of absorbable folic acid daily. Women of childbearing age should take an additional 400 μg/day of folic acid, the amount contained in most multivitamin preparations. Since 1998, the U.S. Food and Drug Administration has required fortification of all enriched cereal grains sold in the United States with 140 μg of folic acid per 100 g of grain. The incidence of neural tube defects in the United States, which had been declining for decades, has fallen an additional 25% since fortification of cereals began.
Folic acid is well absorbed from the jejunum, and oral supplementation is effective both in preventing and in treating megaloblastic anemia associated with folic acid deficiency. This disorder often results from insufficient folic acid intake in the diet, but it can also result from impaired folic acid absorption, such as that seen in patients with alcoholism and certain malabsorption syndromes. In patients with megaloblastic anemia, vitamin B12 deficiency must be ruled out before treatment with folic acid is begun. This is because treatment with folic acid may partly correct the anemia caused by a vitamin B12 deficiency but will not correct other problems associated with it. Irreversible neurologic damage can occur if a B12 deficiency is incorrectly treated with folic acid.
Several drugs can contribute to folate deficiency. These include chemotherapeutic folate reductase inhibitors, such as trimethoprim, pyrimethamine, and methotrexate (see Chapters 40, 44, and 45). Other drugs inhibit folate absorption, including cholestyramine and certain anticonvulsant drugs (e.g., phenytoin).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


