Second-line drugs are reserved to treat patients infected with organisms that are resistant to first-line drugs. They include rifabutin and rifapentine (other derivatives of rifamycin), fluoroquinolone drugs (see Chapter 40), cycloserine, capreomycin, ethionamide, amikacin, and aminosalicylic acid. Most of the second-line drugs are not discussed further in this chapter.
In persons who have had a relapse of TB after earlier treatment, the choice of drugs is guided by in vitro susceptibility of the infecting mycobacterial strain.
Isoniazid
After studies showed that nicotinic acid had a weak antitubercular effect, investigators tested many nicotinic acid derivatives. Isoniazid, also known as isonicotinic acid hydrazide, was found to be the most active derivative and subsequently became available for clinical use. The introduction of isoniazid in the early 1960s revolutionized the treatment of this disease.
PHARMACOKINETICS
Isoniazid is usually given orally and is well absorbed from the gut. The drug is widely distributed to tissues and reaches intracellular concentrations sufficiently high to be effective against organisms inside cells and caseous lesions.
Isoniazid is extensively metabolized, and the parent compound and its metabolites are excreted in the urine. The primary metabolite, acetylisoniazid, is formed by conjugation of acetate with isoniazid in a reaction catalyzed by acetyltransferase, an enzyme whose activity is genetically determined. Slow acetylation is an autosomal recessive trait, and persons with the slow phenotype are homozygous for the slow allele. Persons with the fast phenotype are either heterozygous or autosomal dominant. Because of the different rates of acetylation of isoniazid, persons with the fast phenotype have lower plasma isoniazid concentrations than do persons with the slow phenotype (Fig. 41–1)
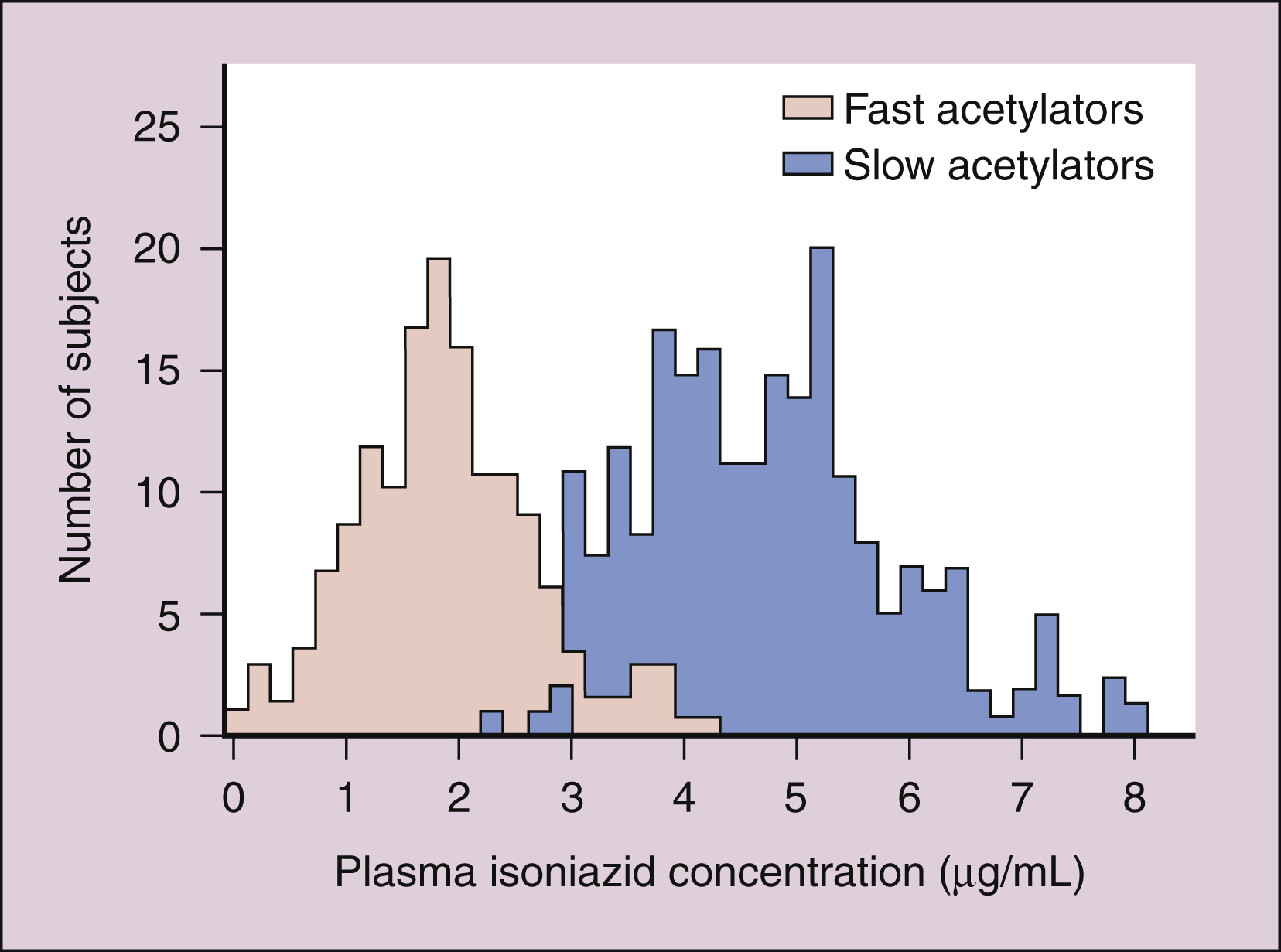
Figure 41–1 Bimodal distribution of plasma isoniazid concentrations. Plasma concentrations of isoniazid were measured 2 hours after the administration of a single 300-mg dose of isoniazid to each member of a general population of human subjects. Subjects with the fast acetylation phenotype showed lower plasma drug concentrations than did subjects with the slow acetylation phenotype.
The prevalence of phenotypes varies from population to population. The slow phenotype predominates in some Middle Eastern populations, whereas the fast phenotype predominates in Japanese populations. In the United States, about half the population exhibits each phenotype.
A small amount of acetylisoniazid is converted to isonicotinic acid and acetylhydrazine. Investigators believe that acetylhydrazine is responsible for the hepatic toxicity of the drug.
MECHANISM
Isoniazid acts by inhibiting the synthesis of mycolic acid. This acid is the mycobacterial cell wall component responsible for the acid-fast staining property of mycobacteria.
Isoniazid is activated by mycobacterial catalase-peroxidase, an enzyme encoded by the katG gene. The hydrazine moiety of isoniazid reduces the ferric component of catalase-peroxidase, and the reduced (ferrous) enzyme combines with oxygen to form an oxyferrous enzyme complex. This complex ultimately interacts with the target enzyme, a long-chain enoyl reductase involved in the synthesis of mycolic acid.
SPECTRUM AND INDICATIONS
Isoniazid is bactericidal against sensitive strains of M. tuberculosis and some strains of M. kansasii. It has little activity against M. avium-intracellulare and is not active against M. leprae or against most other bacteria.
Isoniazid is part of the standard four-drug regimen for TB for persons without known or suspected resistance to isoniazid (Table 41–2) These drugs are believed to kill different populations of TB bacilli, which include rapidly growing organisms and persistent nongrowing (stationary phase) bacteria. Isoniazid, together with rifampin and pyrazinamide, eradicates rapidly growing organisms during the first 2 months of therapy. During the next 4 months of treatment, isoniazid and rifampin act against persistent bacteria that revert to actively growing forms.
Table 41–2 Regimens for Treating Mycobacterial Infections
| Situation | Preferred Therapy | Alternative Therapy |
|---|---|---|
| Prevention of tuberculosis in neonates and children <5 years of age who are exposed to tuberculosis | Isoniazid 10 mg/kg/day for 3 months | |
| Latent tuberculosis (formerly called prophylaxis) | Isoniazid for 9 months (adults: 5 mg/kg/day; children: 10 mg/kg/day) | Rifampin for 4 months (for isoniazid resistant strains) |
| Active Tuberculosis | ||
| Isoniazid resistance <4% | Isoniazid, rifampin, pyrazinamide, and ethambutol for 6 months (up to 9 months for HIV-positive) | Isoniazid, rifampin, ethambutol for 9 months |
| Isoniazid resistance >4% | Rifampin, pyrazinamide, ethambutol ± a fluoroquinolone for 6 months | |
| Rifampin resistance | Isoniazid, ethambutol, and a fluoroquinolone for 18–24 months, with pyrazinamide for 2 months | |
| Isoniazid and rifampin resistance | A fluoroquinolone, pyrazinamide, ethambutol, and amikacin for 18–24 months | |
| Mycobacterium avium-intracellulare prophylaxis | Azithromycin weekly or clarithromycin twice daily | Rifabutin |
| M. avium-intracellulare treatment | Clarithromycin or azithromycin + ethambutol + rifabutin | Preferred therapy + ciprofloxacin and/or amikacin |
| Leprosy | ||
| Tuberculoid | Dapsone + rifampin | |
| Lepromatous | Dapsone + rifampin + clofazimine |
HIV = human immunodeficiency virus.
Isoniazid is also given to treat latent tuberculosis (formerly called prophylaxis) in persons with a positive reaction to the tuberculin skin test and who meet one of the following criteria: human immunodeficiency virus (HIV) positive; recently infected (conversion from a negative to a positive tuberculin skin test in the past 2 years); chest x-ray study showing nonprogressive tuberculous disease; predisposing conditions including illicit injected drug use, diabetes mellitus, immunosuppression, or certain diseases. The preferred duration of treatment for latent TB is 9 months, but 6 months may be sufficient in some cases. Rifampin can be used for prophylaxis if isoniazid is contraindicated or if the mycobacterial strain is known to be resistant to isoniazid (see Table 41–2).
Isoniazid is given to prevent TB in neonates and children who have had close contact with persons in whom active TB was recently diagnosed.
BACTERIAL RESISTANCE
Resistance to isoniazid is increasingly prevalent. This resistance is mediated primarily by mutations of the katG gene, which result in loss of the catalase-peroxidase enzyme required for activation of isoniazid.
ADVERSE EFFECTS
Isoniazid is fairly well tolerated by most patients, but it causes elevation of serum transaminase levels and potentially life-threatening hepatitis in some individuals. The risk of developing hepatitis during isoniazid therapy is low in persons under 35 years of age, is moderate in persons between 35 and 50 years of age, and is highest in persons over 50 years of age. Isoniazid treatment of latent TB (formerly called prophylaxis), however, appears to have a positive risk-benefit ratio in patients over 35 years of age if they are monitored appropriately for hepatotoxicity. Patients who have TB and are being treated with isoniazid should have their serum transaminase levels monitored periodically and should be told to inform their health care provider if they develop symptoms of hepatitis.
Isoniazid can also cause peripheral neuritis, with symptoms including paresthesias and numbness of the fingers and toes. This adverse effect is more likely to occur in individuals with the slow acetylator phenotype, because they have higher plasma concentrations of isoniazid. Peripheral neuritis is caused by a pyridoxine (vitamin B6) deficiency resulting from direct inactivation of pyridoxine by the drug. It can be prevented or treated by administering pyridoxine supplements to patients who are taking isoniazid.
In rare circumstances, isoniazid causes toxic encephalopathy or seizures. Hematologic abnormalities, such as granulocytosis, anemia, or thrombocytopenia, can occur.
Ethambutol
Ethambutol is a butanol derivative that has bacteriostatic activity against mycobacterial organisms. As shown in Table 41–2, it is used in combination with other drugs to treat TB or M. avium-intracellulare infections.
Ethambutol is administered orally, undergoes hepatic biotransformation, and is excreted in the urine and feces. The drug is generally well tolerated, but it can produce dose-dependent optic neuritis and impaired red-green color discrimination. It can also cause hyperuricemia, gout, hepatitis, and thrombocytopenia.
Pyrazinamide
Pyrazinamide is an important drug in TB therapy because of its more rapid bactericidal action and sterilizing effect compared to other agents. Including pyrazinamide in initial treatment regimens made it possible to reduce the treatment duration to 6 months, whereas other therapies required 9 to 12 months. Pyrazinamide is usually given in combination with isoniazid, rifampin, and ethambutol (see Table 41–2).
Pyrazinamide is a nicotinamide derivative that is converted to pyrazinoic acid by susceptible mycobacteria. Pyrazinoic acid inhibits the growth of M. tuberculosis, partly by lowering the ambient pH to a level at which the organism can no longer grow.
Pyrazinamide is given orally, is widely distributed to tissues, and is largely converted to pyrazinoic acid in the liver. A small amount of the drug is excreted unchanged in the urine, along with its metabolite. Adverse reactions to pyrazinamide include hyperuricemia, gout, hematologic toxicity, fever, hepatitis, and an increase in the serum iron concentration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


