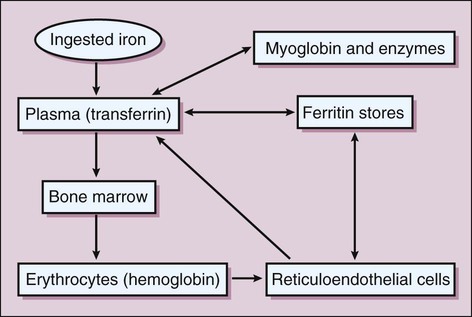
Iron is absorbed from the intestines into the circulation, where it is bound to transferrin and transported to various tissues, including the bone marrow and liver. In these tissues, iron is stored as ferritin (Fig. 17-1). In the marrow, iron is incorporated into heme and packaged in new erythrocytes. The erythrocytes circulate in the blood for about 120 days and then are taken up and degraded by reticuloendothelial cells. These cells later return most of the iron to the plasma so that it can be used again in erythropoiesis. Iron is highly conserved by the body, and only small amounts of it are excreted via the intestinal tract.
Hematopoietic Drugs
Drugs
Minerals
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree