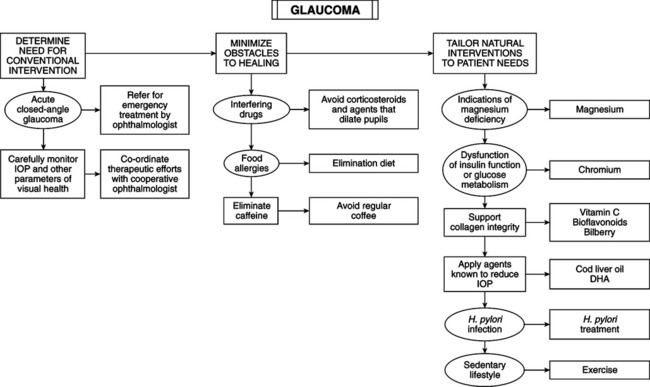
• Three million cases in the United States: 25% undetected; 90% are chronic open-angle type (no consistent anatomic basis for condition). Content and composition of collagen and glaucomatous eye are strongly correlated. • Collagen: most abundant protein in the body, including eye. It provides tissue strength and integrity of cornea, sclera, lamina cribosa, trabecular meshwork, and vitreous. Inborn errors of collagen metabolism (osteogenesis imperfecta, Ehlers-Danlos syndrome, Marfan’s syndrome) have ocular complications: glaucoma, myopia, retinal detachment, ectopia lentis, and blue sclera. Morphologic changes in lamina cribosa (scleral area pierced by optic nerve and blood vessels), trabecular meshwork (connective tissue network the aqueous humor must traverse to reach canal of Schlemm), and papillary blood vessels are found in glaucomatous eyes. Changes may elevate IOP or lead to progressive loss of peripheral vision. • Collagen structure changes: may explain similar peripheral vision loss in patients with normal and elevated IOP, cupping of optic disc even at low IOP, and no apparent anatomic reason for decreased aqueous outflow. • Treatment and prevention depend on reducing IOP and im-proving collagen metabolism in optic disc and trabecular meshwork. • Optic disc is composed of lamina cribosa, optic nerve fibers, and blood vessels. The lamina cribosa is a meshlike network rich in collagen traversed by optic nerves and blood vessels. Collagen changes in lamina cribosa, papillary vessels, and trabecular meshwork precede pressure changes. Intervention must prevent breakdown of ground substance and collagen. • Corticosteroid use should be discouraged in glaucoma because of inhibition of biosynthesis of collagen and glycosaminoglycans (GAGs), causing glaucoma to develop. • Vitamin C: achieving collagen integrity requires optimal tissue ascorbic acid (AA); AA lowers IOP in clinical studies. Daily dose of 0.5 g/kg body weight (single or divided doses) reduces IOP by 16 mm Hg. Near-normal tension was achieved in some patients unresponsive to acetazolamide (a carbonic anhydrase inhibitor) and 2% pilocarpine (a miotic agent). Hypotonic action of AA on eye is long lasting if supplement continued; intravenous administration gives greater initial reduction in IOP. Patient monitoring is necessary to determine required individual dose (2-35 g q.d.). Abdominal discomfort with high doses is common but resolves after 3-4 days. Proposed mechanisms: increased blood osmolarity, diminished production of aqueous fluid by ciliary epithelium, and improved aqueous fluid outflow; AA role in collagen formation may be key. • Bioflavonoids: anthocyanosides (blue-red pigments in berries) elicit AA-sparing effect, improve capillary integrity, and stabilize collagen matrix by preventing free radical damage, inhibiting enzymatic cleavage of collagen matrix, and by cross-linking with collagen fibers directly to form more stable collagen matrix. Vaccinium myrtillus (European bilberry) is rich in these compounds and is used in Europe to reduce myopia, improve nocturnal vision, and reverse diabetic retinopathy. Rutin lowers IOP when used as an adjunct in patients unresponsive to miotics alone. • Allergy: chronic glaucoma has been successfully treated by antiallergy measures. Immediate rise in IOP of up to 20 mm (plus other allergic symptoms) occurred in patients challenged with appropriate allergen. Allergic responses (altered vascular permeability and vasospasm) may cause congestion and edema characteristic of glaucoma. • Magnesium: Channel-blocking drugs benefit some glaucoma patients. Magnesium (Mg) is “nature’s physiologic calcium-channel blocker.” Mg dosed at 121.5 mg b.i.d. for 1 month improved visual fields and peripheral circulation in patients with glaucoma. • Chromium: primary open-angle glaucoma is strongly linked to deficiency of red blood cells, chromium (Cr), and AA and elevated red blood cell vanadium (chromium’s principal antagonist). AA and Cr potentiate insulin receptors that help sustain strong ciliary muscle eye-focusing activity. AA or Cr deficiency is linked with elevated IOP, which stretches normal eye, reducing capacity for focusing power. • Fish oil: cod liver oil reduces IOP dramatically in lab animals in a dose-dependent fashion. Preliminary human trials with docosahexaenoic acid (DHA) are encouraging • Caffeine: Consumption of regular (180 mg caffeine in 200 ml of coffee) by normotensive glaucoma or ocular hypertensive patients elevates IOP. This elevation may be clinically significant.
Glaucoma: Acute (Angle Closure) and Chronic (Open-Angle)
GENERAL CONSIDERATIONS
THERAPEUTIC CONSIDERATIONS
Nutrition
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Glaucoma: Acute (Angle Closure) and Chronic (Open-Angle)
