NURSING ALERT
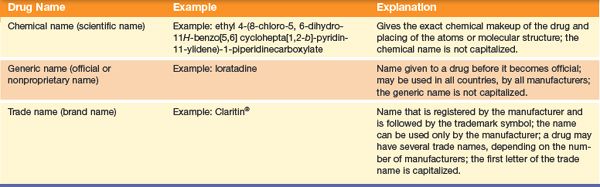
Study the patterns used in the naming of drugs. This may help you to identify names and prevent medication errors. Certain portions of the drug name may be similar in specific drug classes or categories. For example, beta (β)-adrenergic blocking drug names end with “lol.” Atenolol, metoprolol, and propranolol are all antihypertensive drugs from the same category.

Figure 1.1 Example of a prescription form.
Prescription Drugs
Prescription drugs, also called legend drugs, are the largest category of drugs. Prescription drugs are prescribed by a licensed health care provider. The prescription (Fig. 1.1) contains the name of the drug, the dosage, the method and times of administration, and the signature of the licensed health care provider prescribing the drug.
Prescription drugs are designated by the federal government as potentially harmful unless their use is supervised by a licensed health care provider, such as a nurse practitioner, physician, or dentist. Supervision is important because, although these drugs have been tested for safety and therapeutic effect, prescription drugs may cause different reactions in some individuals.
In institutional settings, the nurse administers the drug and monitors the patient for therapeutic effect and adverse reactions. Some drugs have the potential to be toxic (harmful). You will play a critical role in evaluating the patient for toxic effects. When these drugs are prescribed to be taken at home, you will provide patient and family education about the drug.
Nonprescription Drugs
Nonprescription drugs are designated by the FDA as safe (when taken as directed) and can be obtained without a prescription. These drugs are also referred to as over-the-counter (OTC) drugs and may be purchased in a variety of settings, such as a pharmacy, drugstore, or the local supermarket. Over-the-counter drugs include those given for symptoms of the common cold, minor aches and pains, constipation, diarrhea, and heartburn.
These drugs are not without risk and may produce adverse reactions. For example, acetylsalicylic acid, commonly known as aspirin, is potentially harmful and can cause gastrointestinal (GI) bleeding and salicylism (see Chapter 13). Labeling requirements give the consumer important information regarding the drug, dosage, contraindications, precautions, and adverse reactions. Consumers are urged to read the directions carefully before taking OTC drugs.
Controlled Substances
Controlled substances are the most carefully monitored of all drugs. These drugs have a high potential for abuse and may cause physical or psychological dependency. Physical dependency is the habitual use of a drug, in which negative physical withdrawal symptoms result from abrupt discontinuation; it is the body’s dependence on repeated administration of a drug. Psychological dependency is a compulsion or craving to use a substance to obtain a pleasurable experience; it is the mind’s desire for the repeated administration of a drug. One type of dependency may lead to the other.
The Controlled Substances Act of 1970 established a schedule, or classification system, for drugs with abuse potential. The act regulates the manufacture, distribution, and dispensing of these drugs. The Controlled Substances Act divides drugs into five schedules, based on their potential for abuse and physical and psychological dependence. Appendix A describes the five schedules.
Prescription practices of the primary health care provider for controlled substances are monitored by the Drug Enforcement Agency (DEA). Under federal law, limited quantities of certain schedule V drugs may be purchased without a prescription, with the purchase recorded by the dispensing pharmacist. In some cases, state laws are more restrictive than federal laws and impose additional requirements for the sale and distribution of controlled substances. In hospitals or other agencies that dispense controlled substances, the scheduled drugs are counted every 8 to 12 hours to account for each injectable, tablet, or other form of the drug. Any discrepancy in the number of drugs must be investigated and explained immediately.
Drug Development
Drug development is a long and arduous process that can take from 7 to 12 years, and sometimes longer. The FDA has the responsibility for approving new drugs and monitoring drugs currently in use for adverse or toxic reactions. The development of a new drug is divided into the pre-FDA phase and the FDA phase. During the pre-FDA phase, a manufacturer conducts in vitro testing (testing in an artificial environment, such as a test tube) using animal and human cells to discover new drugs. This testing is followed by studies in live animals. The manufacturer then makes application to the FDA for Investigational New Drug (IND) status.
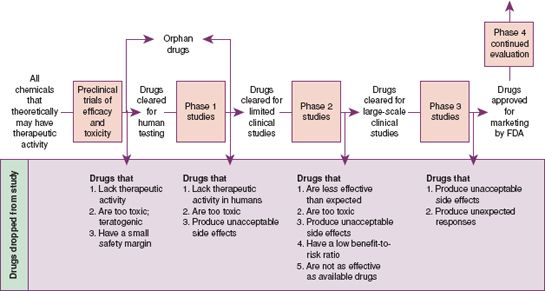
Next, clinical (i.e., human) testing of the new drug begins. Clinical testing involves three phases, with each phase involving a larger number of people (Fig. 1.2). All effects, both pharmacologic and biologic, are noted. Phase 1 involves 20 to 100 individuals who are healthy volunteers. If Phase 1 studies are successful, the testing moves to Phase 2, in which tests are performed on people who have the disease or condition for which the drug is thought to be effective. If those results are positive, the testing progresses to Phase 3, in which the drug is given to large numbers of patients in medical research centers to provide information about adverse reactions. Phase 3 studies offer additional information on dosing and safety. Clinical trial studies can extend for many years.
A New Drug Application (NDA) is submitted after the investigation of the drug in Phases 1, 2, and 3 is complete and the drug is found to be safe and effective. With the NDA, the manufacturer submits all data collected concerning the drug during the clinical trials. A panel of experts, including pharmacologists, chemists, physicians, and other professionals, reviews the application and makes a recommendation to the FDA. The FDA then either approves or disapproves the drug for use.
After FDA approval, continued surveillance is done to ensure safety. Postmarketing surveillance (Phase 4) occurs after the manufacturer places the drug on the market. During this surveillance, an ongoing review of the drug occurs with particular attention given to adverse reactions. Health care providers are encouraged to help with this surveillance by reporting adverse effects of drugs to the FDA by using MedWatch (Display 1.1) or the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program (MERP).
Display 1.1 MedWatch and Reporting Adverse Events
• The U.S. Food and Drug Administration (FDA) established a safety information and adverse event reporting program called MedWatch, by which nurses or other health care providers can learn about or report observations of serious adverse drug effects. Anyone can access the website (http://www.fda.gov/medwatch/index.html) to obtain safety alerts on drugs, devices, or dietary supplements.
• A drug must be used and studied for many years before all of the adverse reactions are identified. Nurses play an important role in monitoring for adverse reactions. The website provides a standardized form for reporting, which can be submitted electronically or downloaded, filled out, and mailed/faxed in to the program. It is important to submit reports, even if there is uncertainty about the cause–effect relationship. The FDA protects the identity of those who voluntarily report adverse reactions.
• The FDA considers serious adverse reactions those that may result in death, life-threatening illness, hospitalization, or disability or those that may require medical or surgical intervention. This form also is used to report an undesirable experience associated with the use of medical products (e.g., latex gloves, pacemakers, infusion pumps, anaphylaxis, blood, blood components).
Special Food and Drug Administration Programs
Although it takes considerable time for most drugs to get FDA approval, the FDA has special programs to meet different needs. Examples of these special programs include the orphan drug program and accelerated programs for urgent needs.
Orphan Drug Program
The Orphan Drug Act of 1983 was passed to encourage the development and marketing of products used to treat rare diseases. The act defines a rare disease as a condition affecting fewer than 200,000 individuals in the United States or a condition affecting more than 200,000 persons in the United States but for which the cost of producing and marketing a drug to treat the condition would not be recovered by sales of the drug. The National Organization of Rare Disorders reports that there are more than 6800 rare disorders that affect approximately 30 million individuals. Examples of rare disorders include multiple myeloma, cystic fibrosis, and phenylketonuria.
The act provides for incentives such as research grants, protocol assistance by the FDA, and special tax credits to encourage manufacturers to develop orphan drugs. If the drug is approved, the manufacturer has 7 years of exclusive marketing rights. More than 360 new drugs have received FDA approval since the law was passed. Examples of orphan drugs include Valortim for anthrax infection, TOL-101 for acute organ transplant rejection, and atiprimod for the treatment of multiple myeloma.
Accelerated Programs
Accelerated approval of drugs is offered by the FDA as a means to make promising products for life-threatening diseases available on the market, based on preliminary evidence and before formal demonstration of patient benefit. The approval that is granted is considered a “provisional approval,” with a written commitment from the pharmaceutical company to complete clinical studies that formally demonstrate patient benefit. This program seeks to make life-saving investigational drugs available before granting final approval to treat diseases that pose a significant health threat to the public. If the drug continues to prove beneficial, the process of approval is accelerated.
One example of a disease that qualified as posing a significant health threat is acquired immunodeficiency syndrome (AIDS). When first diagnosed, because AIDS was so devastating to the individuals affected and because of the danger the disease posed to public health, the FDA and pharmaceutical companies worked together to shorten the IND approval process for some drugs that show promise in treating AIDS. This accelerated process allowed primary health care providers to administer medications that indicated positive results in early Phase 1 and 2 clinical trials, rather than wait until final approval was granted.
Drug Activity within the Body
Once in the body, drugs act in certain ways or phases. Oral drugs go through three phases: the pharmaceutic phase, pharmacokinetic phase, and pharmacodynamic phase (Fig. 1.3). Liquid and parenteral drugs (drugs given by injection) go through the latter two phases only.
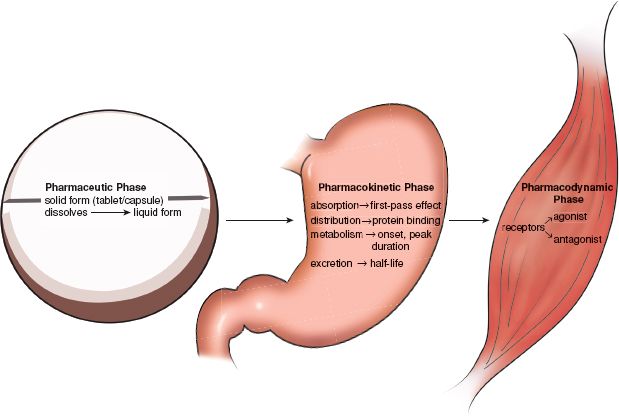
Figure 1.3 Drug activity within the body: pharmaceutic, pharmacokinetic, and pharmacodynamic phases.
Pharmaceutic Phase
In the pharmaceutic phase, the drug dissolves. Drugs must be a soluble liquid to be absorbed. Drugs that are liquid or drugs given by injection (parenteral drugs) are already dissolved and are absorbed quickly. A tablet or capsule (solid forms of a drug) goes through this phase as it disintegrates into small particles and dissolves into the body fluids in the GI tract. Tablets that have an enteric coating or time-release capsules do not disintegrate until they reach the alkaline environment of the small intestine.
Pharmacokinetic Phase
Pharmacokinetics refers to the transportation activity of drugs in the body after administration. These activities include absorption, distribution, metabolism, and excretion. Subcomponents of these pharmacokinetic activities include transport, first-pass effect during absorption, and half-life during excretion of the drug.
Absorption
Absorption involves moving the drug from the site of administration into the body fluids and is the process by which a drug is made available for use in the body. It occurs after dissolution of a solid form of the drug or after the administration of a liquid or parenteral drug. In this process, the drug particles in the GI tract are moved into the body fluids. This movement can be accomplished in several ways:
- Active transport—cellular energy is used to move the drug from an area of low concentration to one of high concentration.
- Passive transport—no cellular energy is used as the drug moves from an area of high concentration to an area of low concentration (small molecules diffuse across the cell membrane).
- Pinocytosis—cells engulf the drug particle (the cell forms a vesicle to transport the drug into the inner cell).
Several factors influence the rate of absorption, including the route of administration, the solubility of the drug, and specific conditions of the body’s tissues. The most rapid route of drug absorption occurs when the drug is given by the intravenous route. Absorption occurs more slowly when the drug is administered orally, intramuscularly, or subcutaneously. This is because the complex membranes of the GI mucosal layers, muscle, and skin delay drug passage. Bodily conditions such as lipodystrophy (the atrophy of subcutaneous tissue from repeated subcutaneous injections) inhibits absorption of a drug given in the affected site.
The first-pass effect may also affect absorption. When a drug is absorbed by the small intestine, it travels to the liver before being released to circulate within the rest of the body. The liver may metabolize a significant amount of the drug before releasing it into the body. When the drug is released into the circulation from the liver, the remaining amount of active drug may not be enough to produce a therapeutic effect, and the patient will need a higher dosage.
Distribution
The systemic circulation transports and distributes drugs to various body tissues or target sites. Distribution of an absorbed drug in the body depends on protein binding, blood flow, and solubility.
When a drug travels through the blood, it comes into contact with proteins such as the plasma protein albumin. The drug can remain free in the circulation or bind to the protein. Only free drugs can produce a therapeutic effect. Drugs bound to protein are pharmacologically inactive. Only when the protein molecules release the drug can the drug diffuse into the tissues, interact with receptors, and produce a therapeutic effect. A drug is said to be highly protein bound when more than 80% of the circulating drug is bound to protein.
A drug is distributed quickly to areas with a large blood supply, such as the heart, liver, and kidneys. In other areas, such as the internal organs, skin, and muscle, distribution of the drug occurs more slowly.
Solubility, or the drug’s ability to cross the cell membrane, affects its distribution. Lipid-soluble drugs easily cross the cell membrane, whereas water-soluble drugs do not.
Metabolism
Metabolism, also called biotransformation, is the process by which the body changes a drug to a more or less active form that can be excreted. Usually the resulting form is a metabolite (an inactive form of the original drug). In some drugs, one or more of the metabolites may have some drug activity. Metabolites may undergo further metabolism or may be excreted from the body unchanged. Most drugs are metabolized by the liver, although the kidneys, lungs, plasma, and intestinal mucosa also aid in the metabolism of drugs.
Excretion
The elimination of drugs from the body is called excretion. After the liver renders drugs inactive, the kidney excretes the inactive compounds from the body. Also, some drugs are excreted unchanged by the kidney without liver involvement. Patients with kidney disease may require a dosage reduction and careful monitoring of kidney function. Children have immature kidney function and may require dosage reduction and kidney function tests. Similarly, older adults have diminished kidney function and require careful monitoring and lower dosages. Other drugs are eliminated in sweat, breast milk, or breath, or by the GI tract through the feces.
Half-life refers to the time required for the body to eliminate 50% of the drug. Knowledge of the half-life of a drug is important in planning the frequency of dosing. Drugs with a short half-life (2 to 4 hours) need to be administered frequently, whereas drugs with a long half-life (21 to 24 hours) require less frequent administration. For example, digoxin (Lanoxin) has a long half-life (36 hours) and requires once-daily dosing. However, aspirin has a short half-life and requires frequent dosing. It takes five to six half-lives to eliminate approximately 98% of a drug from the body. Although half-life is fairly stable, patients with liver or kidney disease may have problems excreting a drug. Difficulty in excreting a drug increases the half-life and increases the risk of toxicity, because these organs do not remove the substances and the drug remains in the body longer. Older patients or patients with impaired kidney or liver function require frequent diagnostic tests measuring renal or hepatic function.
Onset, Peak, and Duration
The therapeutic effect of a drug determines the timing of drug administration. Three pharmacokinetic factors are important when considering how a drug acts in the body:
- Onset of action—time between administration of the drug and onset of its therapeutic effect
- Peak concentration—when absorption rate equals the elimination rate (not always the time of peak response)
- Duration of action—length of time the drug produces a therapeutic effect
These factors are taken into consideration when determining the dose schedule of a specific drug. This ensures that proper blood levels are maintained in the body for the drug to work properly.
Pharmacodynamic Phase
Pharmacodynamics is the study of the drug mechanisms that produce biochemical or physiologic changes in the body. Pharmacodynamics deals with the drug’s action and effect in the body. After administration, most drugs enter the systemic circulation and expose almost all body tissues to possible effects of the drug. This exposure in all tissue causes the drug to produce more than one effect in the body. The primary effect of a drug is the desired or therapeutic effect. Secondary effects are all other effects, desirable or undesirable, produced by the drug.
Most drugs have an affinity for certain organs or tissues and exert their greatest action at the cellular level on those specific areas, which are called target sites. A drug exerts its action by one of two main mechanisms:
1. Alteration in cellular function
2. Alteration in cellular environment
Alteration in Cellular Function
Most drugs act on the body by altering cellular function. A drug cannot completely change the function of a cell, but it can alter its function. A drug that alters cellular function can increase or decrease certain physiologic functions, such as increasing heart rate, decreasing blood pressure, or increasing urine output.
Receptor-Mediated Drug Action
Many drugs act through drug–receptor interaction. The function of a cell is altered when a drug interacts with a receptor. This occurs when a drug molecule selectively joins with a reactive site—the receptor—on the surface of a cell. When a drug binds to and interacts with the receptor, a pharmacologic response occurs.
An agonist is a drug that binds with a receptor and stimulates the receptor to produce a therapeutic response. An antagonist is a drug that joins with receptors but does not stimulate the receptors. The therapeutic action in this case consists of blocking the receptor’s function.
Receptor-Mediated Drug Effects
The number of available receptor sites influences the effects of a drug. When only a few receptor sites are occupied, although many sites are available, the response will be small. When the drug dose is increased, more receptor sites are used, and the response increases. When only a few receptor sites are available, the response does not increase when more of the drug is administered. However, not all receptors on a cell need to be occupied for a drug to be effective. Some extremely potent drugs are effective even when the drug occupies few receptor sites.
Alteration in Cellular Environment
Some drugs act on the body by changing the cellular environment, either physically or chemically. Physical changes in the cellular environment include changes in osmotic pressure, lubrication, absorption, or the conditions on the surface of the cell membrane.
An example of a drug that changes osmotic pressure is mannitol, which produces a change in the osmotic pressure in brain cells, causing a reduction in cerebral edema. A drug that acts by altering the cellular environment by lubrication is sunscreen. An example of a drug that acts by altering absorption is activated charcoal, which is administered orally to absorb a toxic chemical ingested into the GI tract. The stool softener docusate is an example of a drug that acts by altering the surface of the cellular membrane. Docusate has emulsifying and lubricating activity that lowers the surface tension in the cells of the bowel, permitting water and fats to enter the stool. This softens the fecal mass, allowing easier passage of the stool.
Chemical changes in the cellular environment include inactivation of cellular functions or alteration of the chemical components of body fluid, such as a change in the pH. For example, antacids neutralize gastric acidity in patients with peptic ulcers.
Other drugs, such as some anticancer drugs and some antibiotics, have as their main site of action the cell membrane and various cellular processes. They incorporate themselves into the normal metabolic processes of the cell and cause the formation of a defective final product, such as a weakened cell wall, which results in cell death, or reduce a needed energy substrate that leads to cell starvation and death.
Pharmacogenomics
Most pharmacodynamic mechanisms deal with principles that affect each cell in the same way, whereas pharmacogenomics is the study of how people’s responses to medications are variable due to individual genetic variation. In other words, the genetic makeup of a person can affect the pharmacodynamics of a drug. This discovery was made during the Human Genome Project when many scientists were able to determine the different components of the human genetic code. Pharmacogenetics is the creation of individualized drug therapy that allows for the best choice and dose of drugs.
Drug Use, Pregnancy, and Lactation
The use of any medication (prescription or nonprescription) carries a risk of causing birth defects in the developing fetus. Drugs administered to pregnant women, particularly during the first trimester (3 months), may have teratogenic effects. A teratogen is any substance that causes abnormal development of the fetus, often leading to severe deformation. Some drugs are classified as teratogens.
To prevent teratogenic effects, the FDA has established five categories suggesting the potential of a drug for causing birth defects (Appendix A). Information regarding the pregnancy category of a specific drug is found in reliable drug literature, such as the inserts accompanying drugs and approved drug references. In general, most drugs are contraindicated during pregnancy and lactation unless the potential benefits of taking the drug outweigh the risks to the fetus or the infant.
During pregnancy, no woman should consider taking any drug, legal or illegal, prescription or nonprescription, unless the drug is prescribed or recommended by the primary health care provider. Smoking or drinking any type of alcoholic beverage also carries risks, such as low birth weight, premature birth, and fetal alcohol syndrome. Children born of mothers using addictive drugs, such as cocaine or heroin, often are born with an addiction to the drug abused by the mother. Women who are pregnant should also be very careful about the use of herbal supplements because these products can act like drugs. Women should not take an herbal supplement without discussing it first with her primary health care provider.
When a mother breastfeeds, her child has a risk of exposure to harmful medications. A number of drugs can be excreted in breast milk. Therefore, if a mother is lactating (breastfeeding), some of the drug she is taking may be ingested and absorbed by the infant or breastfeeding child. It is important for both mothers and nurses to know the potential of exposure to a breastfeeding child when the mother is taking a drug.
The National Library of Medicine provides a free online database with information on drugs and lactation called LactMed (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT). This website is geared to the health care practitioner and nursing mother and contains over 450 drug records. It includes information such as maternal levels in breast milk, infant levels in blood, and potential effects in breastfeeding infants. A pharmacist, Dr. Thomas Hale, from Texas Tech University has developed a system of lactation risk categories similar to that of the FDA pregnancy risk categories for drugs. Drugs are assigned an L1 to L5 risk according to the drug’s transmission in breast milk and the effect it may have on the child. Hale’s listing of certain drugs may differ from those published by organizations such as the American Academy of Pediatrics, yet it is a good starting point for discussion with mothers who are breastfeeding.
Drug Reactions
Drugs produce many reactions in the body. The following sections discuss adverse drug reactions, allergic drug reactions, drug idiosyncrasy, drug tolerance, cumulative drug effect, and toxic reactions.
Adverse Drug Reactions
Patients may experience one or more adverse reactions or side effects when they are given a drug. Adverse reactions are undesirable drug effects. Adverse reactions may be common or may occur infrequently. They may be mild, severe, or life-threatening. They may occur after the first dose, after a few doses, or after many doses. Often, an adverse reaction is unpredictable, although some drugs are known to cause certain adverse reactions in many patients. For example, drugs used in treating cancer are very toxic and are known to produce adverse reactions in many patients receiving them. Other drugs produce adverse reactions in fewer patients. Some adverse reactions are predictable, but many adverse drug reactions occur without warning.
Some texts use both the terms side effects and adverse reactions, using side effects to explain mild, common, and nontoxic reactions and adverse reactions to describe more severe and life-threatening reactions. For the purposes of this text, only the term adverse reaction is used, with the understanding that these reactions may be mild, severe, or life-threatening.
Allergic Drug Reactions
An allergic reaction is an immediate hypersensitivity reaction. Allergy to a drug usually begins to occur after more than one dose of the drug is given. On occasion, the nurse may observe an allergic reaction the first time a drug is given, because the patient has received or taken the drug in the past.
A drug allergy occurs because the individual’s immune system responds to the drug as a foreign substance called an antigen. When the body responds to the drug as an antigen, a series of events occurs in an attempt to render the invader harmless. Lymphocytes respond by forming antibodies (protein substances that protect against antigens). Common allergic reactions occur when the individual’s immune system responds aggressively to the antigen. Chemical mediators released during the allergic reaction produce symptoms ranging from mild to life-threatening.
Even a mild allergic reaction produces serious effects if it goes unnoticed and the drug is given again. Any indication of an allergic reaction is reported to the primary health care provider before the next dose of the drug is given. Serious allergic reactions require contacting the primary health care provider immediately, because emergency treatment may be necessary.
Some allergic reactions occur within minutes (even seconds) after the drug is given; others may be delayed for hours or days. Allergic reactions that occur immediately often are the most serious.
Allergic reactions are manifested by a variety of signs and symptoms observed by the nurse or reported by the patient. Examples of some allergic symptoms include itching, various types of skin rashes, and hives (urticaria). Other symptoms include difficulty breathing, wheezing, cyanosis, a sudden loss of consciousness, and swelling of the eyes, lips, or tongue.
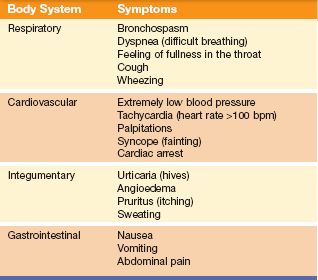
Anaphylactic shock is an extremely serious allergic drug reaction that usually occurs shortly after the administration of a drug to which the individual is sensitive. This type of allergic reaction requires immediate medical attention. Symptoms of anaphylactic shock are listed in Table 1.2.
All or only some of these symptoms may be present. Anaphylactic shock can be fatal if the symptoms are not identified and treated immediately. Treatment is to raise the blood pressure, improve breathing, restore cardiac function, and treat other symptoms as they occur. Epinephrine (adrenalin) may be given by subcutaneous injection in the upper extremity or thigh and may be followed by a continuous intravenous infusion. Hypotension and shock may be treated with fluids and vasopressors. Bronchodilators are given to relax the smooth muscles of the bronchial tubes. Antihistamines and corticosteroids may also be given to treat urticaria and angioedema (swelling).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree