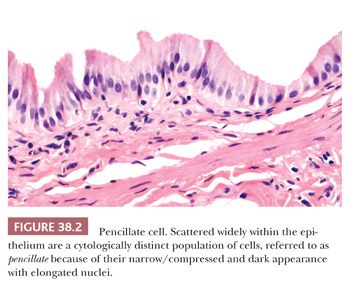
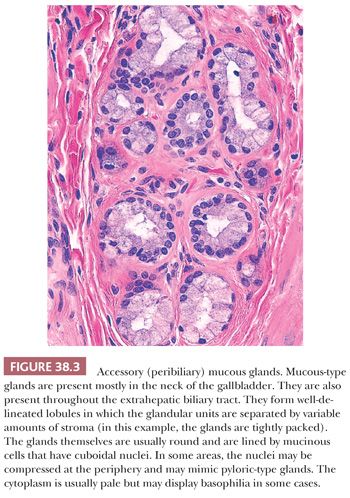
The mucosal layer is thrown into variably sized branching folds, which are more prominent when the organ is contracted. The surface epithelium consists of a single layer of tall columnar cells containing abundant pale, eosinophilic, apical cytoplasm and basally located nuclei with inconspicuous nucleoli. A seemingly separate minor population of cells of debatable nature, referred to as pencil-like because of their narrow, contracted appearance (Fig. 38.2), are scattered individually amongst the usual columnar cells. These two cell types have only a few enzymatic and ultrastructural differences. Few basal cells, which are oval-shaped and inconspicuous, run parallel to and are located just above the basement membrane. A few intraepithelial T lymphocytes may be found. Goblet cells and melanocytes are absent. In the gallbladder, true glands are present only in the neck (Fig. 38.3). These tubuloalveolar mucus glands have some histologic resemblance to, but differ histochemically and ultrastructurally from, the pyloric metaplasia that is commonly seen throughout the gland in chronic cholecystitis and cholelithiasis. In diseased gallbladders, a spectrum of gastric and intestinal metaplasia may appear, including argyrophilic and argentaffin cells, which are not present normally.
The lamina propria lies between the surface epithelium and muscularis and fills the cores of the mucosal folds. It is composed of loose connective tissue and small numbers of lymphocytes, plasma cells, and macrophages. Neutrophils and eosinophils are normally absent. Nerve fibers, small blood vessels, and lymphatics are present.
The layering of the gallbladder is very different from the rest of the gastrointestinal (GI) tract, which becomes important in staging of tumors (Fig. 38.1) (9). There is no muscularis mucosa in the gallbladder. The only muscular layer present is called the tunica muscularis and it consists of loosely arranged bundles of smooth muscle. It does not form well-defined layers as seen in the tubular GI tract. The junction of the muscularis and lamina propria is highly irregular; some muscle fibers extend upward toward the epithelium. Mucosal invaginations (diverticula), referred to as Aschoff-Rokitansky sinuses (10), may be seen in normal gallbladders (in up to 40% of autopsies) but are much more pronounced in inflamed gallbladders.
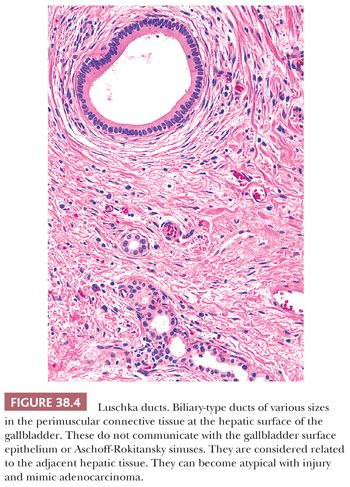
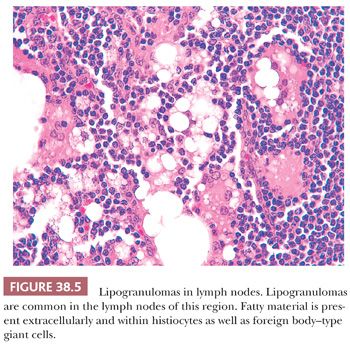
The perimuscular connective tissue (also called subserosa or adventitia) contains variable amounts of fibroelastic and adipose tissue as well as nerves, vessels, ganglia, and paraganglia (10). Bile ductules, known as Luschka ducts (Fig. 38.4), which are thought to be remnants of the embryonic primordium of the liver, may be seen in the perimuscular connective tissue, especially adjacent to the liver (10). They have been reported in 10% of cholecystectomy specimens and may occasionally measure up to 1 to 2 mm. They are lined by a cuboidal type of biliary epithelium, similar to the intrahepatic bile ductules and are surrounded by a collar of fibrous tissue (Fig. 38.4). They may appear pseudoinfiltrative in cases with cholecystitis (11). They do not communicate with Aschoff-Rokitansky sinuses. Lymph nodes in the region may contain lipogranulomas (Fig. 38.5) as is the case for any of the intra-abdominal lymph nodes located above the ligament of teres.
Histochemically, the epithelial cells contain mainly acidic sulfomucin in contrast with those of metaplastic glands (12). By immunohistochemistry, the epithelial cells are reactive for both low–molecular-weight keratin and epithelial membrane antigen. Carcinoembryonic antigen expression is confined to the apical surface of the cells. CD117-expressing stromal cells are detectable in the muscle layer, and these are probably analogous to the interstitial cells of Cajal of the GI tract (7).
ROUTINE SPECIMEN PROCESSING
Because the epithelium is quite susceptible to bile-related autolysis, prompt fixation of gallbladders is required. It is advisable to open the gallbladder from the fundus to the cystic duct. In the initial sampling, the ideal approach (13–15) is to take a full slice going from the fundus to the cystic duct margin, which can be divided into three fragments when placing into the cassette. The cystic duct margin should be identified with an ink because this otherwise becomes a major problem if a grossly invisible lesion such as extensive early gallbladder carcinoma (16) is encountered in these sections incidentally. Although uninjured cystic duct is recognizable with its distinct histology, in the cases with pathologic conditions, it may not be distinguishable by microscopic examination alone.
Pericystic duct lymph nodes, if present, should also be sampled. If gallstones are not readily apparent, the bile should be strained to assess for minute stones or “floating” polyps, and the relative viscosity of the bile should be noted in the gross description of the specimen (see discussion under “Cholelithiasis”). Granular debris in the lumen should be carefully evaluated because polypoid tumors, neoplastic or nonneoplastic, often become detached from the surface and have such an appearance.
If polyps or tumors are identified grossly, these should be entirely submitted. It should be kept in mind that more than half of the carcinomas of gallbladder are clinically and grossly inapparent (17–19). Therefore, if any suspicious lesion is identified in the initial sampling, then a revisit and thorough examination of the organ is warranted (20,21). If focal epithelial atypia is encountered in the initial set, then additional two to four blocks would be adequate (22); however, if there is convincing high-grade dysplasia or a process with proven high risk for invasive carcinoma such as hyalinizing cholecystitis (23), then thorough sampling is warranted (20). The deepest penetration of a given tumor is also very difficult to appreciate. Considering that the prognosis of muscle-confined versus muscle-penetrating carcinomas appears to be starkly different (16), it is crucial to revisit the specimen and perform extensive (if not thorough) sampling in cases where the tumor seems to be superficial in the initial sampling. In cases with overt malignancy, in addition to deepest penetration, it is also important to document the involvement of the serosal versus hepatic surfaces of the organ, separately.
If hepatic wedge or segmental resection for gallbladder carcinoma is performed, the extent of tumor penetration into the liver should be documented, as should the status of the hepatic margin. The approach in the further sampling of documentation of pathologic conditions is also discussed in the corresponding sections (20).
CONGENITAL ANOMALIES
Congenital anomalies of the gallbladder are rare (3,24). They include anomalies of number (agenesis, duplication, and triplication), shape/structure (phrygian cap, multiseptate gallbladder), and position, in addition to cysts, diverticula, and heterotopias.
Agenesis and hypoplasia of the gallbladder (25,26) are rare, with a reported incidence of 0.1%. These are usually associated with corresponding anomalies in the cystic duct and other components of extrahepatic bile ducts. Patients with agenesis usually fall into one of three groups: children with multiple congenital abnormalities, asymptomatic adults diagnosed at autopsy, or symptomatic adults with choledocholithiasis. Although agenesis has little clinical significance in childhood, the hypoplastic gallbladder has several important associations. In patients with extrahepatic biliary atresia, only a fibrous cord may remain in the gallbladder fossa. At the microscopic level, the site of the gallbladder consists of compressed, epithelium-lined structures, fibrous tissue, strands of smooth muscle, and an inflammatory infiltrate. Similar findings may be seen in the porta hepatis. A small gallbladder (<2 to 3 cm in length) may be associated with idiopathic neonatal (giant cell) hepatitis, α1-antitrypsin deficiency, and cystic fibrosis. Congenital hypoplasia may be difficult to distinguish from postinflammatory shrinkage. Duplication (27) of the gallbladder is very rare and triplication (28) is even rarer. These are classified according to the arrangement of the cystic ducts. Multiplicate gallbladder may be a rare cause of recurrent acute right upper quadrant pain after cholecystectomy (29).
Diverticula in the gallbladder are mostly acquired as a consequence of inflammation; however, rarely true congenital ones may also occur. These probably arise after incomplete cavitation in the developing gallbladder bud. Unlike the acquired ones, congenital diverticula contain all the normal layers of the fully developed gallbladder. They can occur anywhere in the gallbladder and may measure up to 8 cm.
A variety of congential cysts have been described in the gallbladder. Fundal cyst (30) is probably a diverticulum in which the connection to the lumen proper is cut off by inflammation. Excessively dilated Luschka ducts (Fig. 38.4) may also form macroscopic cysts. Mesothelial cysts have also been described (31).
Multiseptate gallbladder (32) consists of a partitioning of the lumen into 3 to 10 communicating compartments. Patients may develop stones when they become adults. Congenital hourglass gallbladder is another form of septate gallbladder in which there is a centrally located transverse septum associated with constriction in this region.
Malpositions include the so-called floating gallbladder (33) in which the whole of the organ is invested by a serous membrane and is then connected to the liver by a kind of mesentery. The extreme mobility of this floating gallbladder may cause torsion and hemorrhagic infarction (34) and may also lead to hypoplasia of the right hepatic lobe (33). Conversely, some gallbladders are completely embedded in the liver. The latter may be prone to cholelithiasis and infections (35). The gallbladder may also be located in aberrant sites such as the suprahepatic region, retroperitoneum, lesser sac, falciform ligament, or abdominal wall.
A variety of tissue types including GI, pancreatic, hepatic, adrenal, and thyroid have been reported to form ectopic nodules (heterotopia) in the gallbladder (36–40). Only those that give rise to a mass lesion should be classified as heterotopia because some of these tissue types (especially GI and rarely, pancreatic) are also believed to develop as a result of metaplasia secondary to inflammation. Also, before hepatic heterotopia is considered, the possibility of an extension from the liver proper or an accessory lobe needs to be ruled out; the nodule should be anatomically separate from the main organ. Heterotopic pancreatic tissue may be associated with acute pancreatitis confined to the gallbladder (41). Ectopic gastric mucosa may occasionally lead to perforation and hemorrhage. Although ectopic adrenal may be encountered not uncommonly in the abdominal organs, it is exceedingly rare in the gallbladder (39).
Phrygian cap is more a curiosity because of its name than the lesion itself, as it has no significance, provided that the correct diagnosis is made radiologically (42). In fact, it is relatively common (6%); however, it rarely comes to pathologists’ attention. It only becomes detectable if the gallbladder is fixed in the distended state and cut longitudinally. It represents an inversion of the fundus into body of the organ, to which it may become adherent. The name phrygian cap was coined because of the resemblance of the shape to a type of headgear worn originally by Phrygians, an ancient civilization in Asia Minor, now a part of modern Turkey. Picked up subsequently by Western civilizations, the cap later became a symbol of liberty. It recently became repopularized by Smurfs.
CHOLESTEROLOSIS
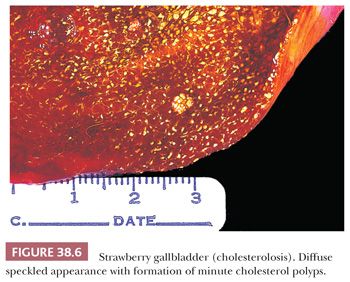
Cholesterolosis (43–45) refers to the accumulation of lipid, particularly cholesterol esters and triglycerides, within the macrophages of the lamina propria and, to a lesser extent, in the epithelium itself. Supersaturation of bile with esterified cholesterol and an abnormal lipid transport across the mucosa are suspected to be the cause for this accumulation (46,47). Cholesterolosis is seen primarily in adults and often in multiparous women. No association with high serum cholesterol levels or with coronary artery disease has been found (48). Generally, it is limited to the gallbladder; the cystic duct is spared. The process may be diffuse or patchy and in some cases become polypoid (“cholesterol polyp”) although a significant proportion of well-established cholesterol polyps are unaccompanied by cholesterolosis. More diffuse examples form linear yellow streaks in the prominences of the ridges, intervened by congested mucosa and are termed strawberry gallbladder (Fig. 38.6). The bile in the lumen is generally dark and thick, with a high concentration of cholesterol and sometimes floating yellow lipid particles (“lipioidic corpuscles”).
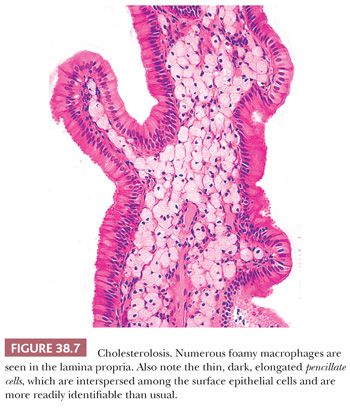
The typical microscopic finding of cholesterolosis is the expansion of the superficial lamina propria by foamy (lipid-filled) macrophages (Fig. 38.7). This leads to a microvillous configuration in the mucosa. Lipid can also be demonstrated in the epithelial cells by special stains. Occasionally, foamy macrophages may also be found deeper within the gallbladder wall. Cholesterolosis is often seen in quiescent gallbladders that are devoid of any significant inflammation or neoplasm, which has led to the speculation that this inverse correlation may be an indicator of a protective environment that cholesterolosis represents, or that it is surrogate of, or induces. Some have also speculated that inflammation may be aiding the resorption of lipid deposits.
CHOLELITHIASIS
Epidemiology
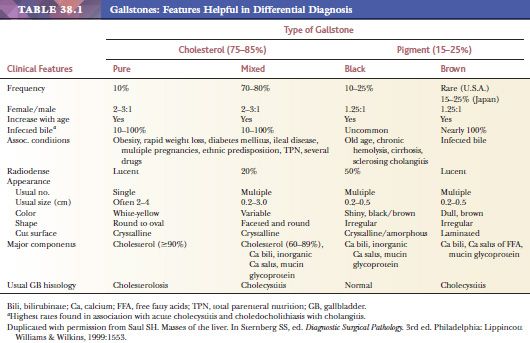
Cholelithiasis is a common disorder (Table 38.1), afflicting 10% to 20% of adult populations in developed countries (49–53). It occurs predominantly in females, and the incidence increases with age, possibly owing to a progressive increase in the secretion of biliary cholesterol. Cholelithiasis is very uncommon in children (54). In addition to the significantly higher incidence in developing countries, there are also ethnic differences: Cholesterol gallstones are seen in 75% of the Native Americans who have migrated from Asia to North America. Dominant lithogenic genes are suspected in these populations (52).
Classification, Pathogenesis, and Morphology
Cholesterol stone formation requires the supersaturation of bile with cholesterol, which results from increased biliary cholesterol output, decreased bile acid synthesis, or both (55,56). Gallbladder hypomotility and mucin hypersecretion promote the precipitation and agglomeration of cholesterol monohydrate crystals into stones. Factors that induce hepatic excretion of cholesterol such as pregnancy, oral contraceptives, anticholesterol medications, obesity, rapid weight loss, hypertriglyceridemia, and total parenteral nutrition (57), as well as those that lead to stasis of the gallbladder (neurogenic, hormonal, or mechanical obstruction), enhance cholesterol-type stone formation. Other conditions such as Crohn disease may lead to cholesterol stones by depleting bile salts (58). Whether diabetes predisposes to gallstone formation is still controversial. Substantial evidence suggests that alcohol intake protects against gallstones (57,59).
The appearance of cholesterol stones varies depending on the cholesterol content (Fig. 38.8). Pure cholesterol stones, which account for 10% of gallstones, are yellow-white, round to ovoid, smooth, have a crystalline or laminated cut surface, and measure up to 4 cm in diameter. Stones with a lower cholesterol content are designated as mixed. Depending on the proportion of calcium carbonate, bilirubin, and phosphates, mixed stones may be lamellated gray-white to black. They are usually smaller, multiple, and faceted or may have laminated cut surfaces and a dark core. More than 80% of cholesterol stones lack calcium carbonate and are hence radiolucent.

Pigment stones (60,61) are the most common worldwide, particularly in Asia, due to parasitic infections (see the following texts), but constitute less than 25% of the cholelithiasis in the United States. By definition, they contain less than 25% to 30% cholesterol. They form as a result of increased unconjugated bilirubin in the bile, which then forms insoluble calcium salts. Factors that increase the amount of unconjugated bilirubin in bile, such as hemolytic syndromes, severe ileal dysfunction, or bypass, lead to pigment stones, in addition to bacterial and parasitic infestation of the biliary tree (61).
Pigment stones can be divided into two types: black and brown. Black stones are black or deep brown, relatively small (2 to 5 mm), resist crushing, have an irregular shiny surface, and on fracturing have a glasslike, featureless appearance. They arise in sterile bile, and most patients have no predisposing conditions except for advanced age. On the other hand, stones associated with chronic hemolytic anemias, cirrhosis, and sclerosing cholangitis are also mostly of this type (62). Many black stones (50% to 75%) contain enough calcium carbonates and phosphates to render them radiopaque. Brown stones are much softer than black ones; have a rough, flaky appearance; and at times may be greasy-appearing. They form in the context of biliary stasis and infection (bacteria, particularly Escherichia coli, and parasites, especially Ascaris lumbricoides and Opisthorchis sinensis). Enzymes produced by these organisms, particularly bacterial phospholipases, liberate free fatty acids which then complex with calcium. Beta-glucuronidases hydrolyze conjugated bilirubin to unconjugated bilirubin, hence leading to stone formation (63). Brown stones are usually radiolucent.
Disappearing gallstones refers to a rare phenomenon in which gallstones vanish, possibly due to spontaneous passage via the common bile duct (CBD) or cholecystoenteric fistula or through spontaneous dissolution. Intramural gallstones refer to those that are embedded in the gallbladder wall, possibly via ulcerated mucosa or Aschoff-Rokitansky sinuses. These intramural gallstones can appear in histologic sections. Floating gallstones is the name given by radiologists to those that float in the bile due to a density gradient between bile and the contents, which may be induced by fasting. Approximately 20% of the gallstones are reported as gas-containing by radiologists; it has been hypothesized but not confirmed that the gas in these gallstones is produced by bacteria.
Clinical Features
Studies have shown that gallbladder stones grow at a rate of approximately 1 to 2 mm a year; the most rapid growth takes place in the first 2 to 3 years, after which their growth stabilizes. The vast majority of gallstones are clinically silent. The chief complaint in symptomatic patients is right upper quadrant pain associated with flatulence and discomfort induced by fatty food. Colicky pain may occur. The diagnosis is often established by ultrasonography, which can easily detect gallstones larger than 2 mm; even “sludge”—the gallstone precursor—can be identified. Ten percent to 25% of gallstones (including 20% of cholesterol stones and 50% of pigment stones) can be visualized on plain abdominal radiographs.
Cholecystectomy, the treatment of choice, is recommended mostly for symptomatic patients. Other indications for surgery include gallstones in children, high cancer-risk populations (Native Americans), patients with sickle cell disease, porcelain gallbladder, or gallstones larger than 3 cm diameter. Nonsurgical options (oral and contact dissolution therapy, extracorporeal shock-wave lithotripsy) are rarely used.
Complications
Gallbladder stones may lead to several complications. The more common and important ones, namely, cholecystitis, hydrops, mucocele, and the association of gallstones with cancer will be discussed in detail in later sections.
Fistulas. Gallstones may lead to internal biliary fistulas (64). Most of these are between the gallbladder and duodenum or colon. These fistulas develop by the formation of inflammatory adhesions and subsequent dissection of the tissue by the stones. The passage of stones into the biliary tree and GI tract, either through these fistulas or through the cystic duct may be uneventful; however, in some cases, it leads to obstruction and related complications in the corresponding sites: choledocholithiasis (with choleductitis); obstructive jaundice; obstruction of the ampulla of Vater and secondary pancreatitis; obstruction of the small intestine, particularly the ileum (“gallstone ileus”) (65); and, rarely, also of the large intestine and appendix. Stones that pass from the CBD through the ampulla of Vater into the GI tract are, in general, too small to cause obstruction. Therefore, it is assumed that the stones that lead to gallstone ileus have entered through a cholecystoenteric fistula, which is often no longer demonstrable at the time of operation. The gallbladder in such cases is usually small, fibrotic, and surrounded by adhesions.
Rarely, a stone in the gallbladder neck or the cystic duct may compress the CBD extrinsically and lead to jaundice (“Mirizzi” syndrome) (66).
HYDROPS AND MUCOCELE
Hydrops is the marked distension of the gallbladder with clear, watery fluid and mucocele by cloudy, mucoid material (67). Mucocele of the gallbladder is a reactive process and should be distinguished from mucinous neoplasms. Together, hydrops and mucocele are said to account for 3% of pathologic conditions of the adult gallbladder but are much less common in our experience. In most cases, they develop as a consequence of obstruction of the cystic duct, generally due to an impacted calculus or, rarely, to slow-growing tumors, fibrosis, or cirrhotic liver nodules. In adults, these changes do not resolve spontaneously and cholecystectomy may be required. In children, however, hydrops is often reversible because it generally develops secondary to infections or inflammation, including Kawasaki syndrome (mucocutaneous lymph node syndrome), Epstein-Barr virus (EBV) infections, Henoch-Schönlein purpura, and others (68). A congenital anomaly in the cystic duct should be excluded. Patients usually present with vomiting and a right upper quadrant mass. Pain and tenderness may also be present.
Grossly, these gallbladders may reach significant sizes. Some weigh up to 2 kg. At the microscopic level, the epithelium is flattened, and varying degrees of Aschoff-Rokitansky sinus development may be seen. Inflammation is usually sparse. In adult cases, because the process is usually chronic and slow-growing, the gallbladder wall is thickened. The muscular layer may be replaced by fibrous tissue. Muciphages may be present in the stroma and should not be misdiagnosed as signet ring cell adenocarcinoma. In pediatric cases, where obstruction is often acute, the wall is generally thin.
ACUTE CHOLECYSTITIS
Definition, Classification, and Pathogenesis
Acute cholecystitis (69,70) is a clinically defined entity, characterized by an abrupt injury of the organ. It does not necessarily imply acute inflammation of the gallbladder, in the sense that the hallmark of acute inflammation, polymorphonuclear leukocytes, is often lacking (71). Nevertheless, it is an acute destructive process typically associated with ischemia, congestion, edema, epithelial denudation, vascular leakage, and fibrinous changes.
The vast majority of acute cholecystitis cases (90% to 95%) are related to gallstones. It usually develops when the stones are lodged in the neck or the cystic duct and cause impaction. This leads to chemical and/or ischemic injury to the mucosa (72). It is postulated that, as a consequence of the obstruction, mucosal phospholipases hydrolyze luminal lecithins to lysolecithins, the inflammatory cascade is activated, and the mucosal protective layer is disrupted, rendering the mucosa vulnerable to the detergent action of bile salts (69,73). Distention of the bladder and disturbance of motility leads to the obstruction of venous flow, thus enhancing the injury. Typically, these events occur in the absence of bacterial invasion. Bacterial infection, however, eventually develops as a complication (a secondary phenomenon) in about 50% of the cases. Aerobes (E. coli, Enterobacter, Enterococcus, Klebsiella) predominate over anaerobes (Clostridium, Peptostreptococcus, Bacteroides) (74).
Acute acalculous cholecystitis (75,76) accounts for about 5% of all cases of acute cholecystitis (77,78). It is regarded separately from calculous cholecystitis. Its precise etiology may not be demonstrable (69,79); however, it is typically seen in the context of severe debilitating conditions such as major trauma, burns, multisystem organ failure, major nonbiliary surgery, cancer, diabetes mellitus, and vasculitides. It may follow systemic infections such as hemolytic streptococcal septicemia or typhoid fever as well as others (Vibrio cholerae, Clostridium, Legionella). Some cases are related to a postpartum state (80); yet a few others are associated with chemical injury to the gallbladder associated with bone marrow transplantation, hepatic arterial chemotherapy, and prolonged intravenous hyperalimentation (81). Regardless of the underlying condition, the main pathogenic event in acute acalculous cholecystitis is thought to be direct ischemic injury, possibly triggered by activated factor XII (82,83). That the gallbladder relies solely on the cystic artery, with no collaterals, is considered an important factor (76). Other possible contributing factors include dehydration and multiple blood transfusions leading to increased pigment load, gallbladder stasis, production of viscous bile, and bacterial contamination with generation of lysolecithins.
There are other forms of acute cholecystitis that may have specific causes other than gallstones. Cytomegalovirus (CMV), cryptosporidia, and microsporidia have been implicated in causing an “acute cholecystitis”–type clinical picture in patients with acquired immunodeficiency syndrome (see “AIDS-Related Cholecystitis”).
Clinical Findings
Acute calculous cholecystitis (69,70) is the primary complication of gallstones and is also the most common reason for emergency cholecystectomy. Some studies imply that the incidence of acute cholecystitis may be declining due to an increase in the rate of elective cholecystectomy performed laparoscopically (84,85). It develops predominantly in the sixth and seventh decades of life and is slightly more common in females. Patients present with the characteristic acute cholecystitis syndrome: upper outer quadrant pain, nausea, vomiting, and, occasionally, mild jaundice. Prominent elevation of bilirubin indicates coexistent choledocholithiasis.
Acute acalculous cholecystitis (75,76) is also seen in late adulthood but also constitutes a higher proportion of pediatric cases with acute cholecystitis. Patients with acalculous cholecystitis may not present with the classical cholecystitis syndrome; they may only have fever or hyperamylasemia. In others, the symptoms are masked by the underlying debilitating condition, and the event becomes more insidious. Cocaine-induced cholecystitis should be considered in a young and otherwise healthy patient if vascular thrombosis is noted, and other portions of the GI tract are affected.
Pathologic Findings
The pathologic features of the gallbladder wall in acute calculous and acute acalculous cholecystitis are similar. Grossly, the gallbladder is often distended and edematous; there is a serosal exudate and the serosa may appear discolored and hemorrhagic. Subserosal vessels are engorged. The wall is often thickened (up to 2 cm) with edema and hemorrhage. Mucosal ulcerations are common and may be extensive. An obstructing stone may be present in the neck or in the cystic duct in calculous cholecystitis. The gallbladder lumen is filled with admixed bile, hemorrhagic material, and/or turbid fluid; this may mimic empyema, but in reality, the material is not pus but a viscous mixture of cholesterol and calcium carbonate.
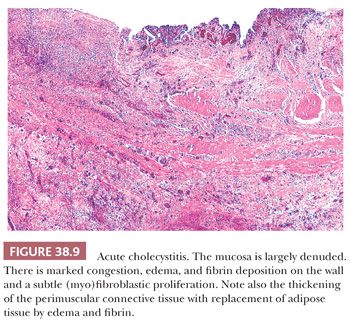
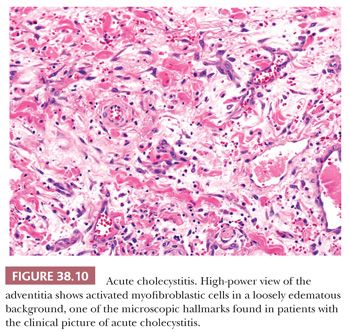
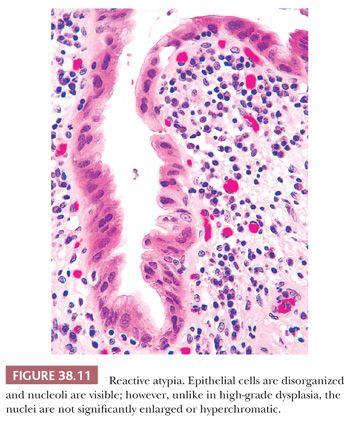
The nature of the microscopic changes depends on the severity and duration of the disease. Acute cholecystitis does not necessarily contain neutrophils; in fact, it often does not unless the gallbladder is secondarily infected. Intraepithelial aggregates of neutrophils may also appear in the mucosa in cases with associated choledocholithiasis. Eosinophils, on the other hand, often accompany acute cholecystitis, presumably in response to the chemical attractant effect of bile contents, in particular, those cases with loss of mucosal integrity. The principal microscopic findings of early, uncomplicated acute cholecystitis are prominent edema, congestion, hemorrhage, and fibrin deposition. The mucosa is often eroded. The overall picture imparts a red appearance to the histologic sections prepared by hematoxylin and eosin. Mucosal ulcers and/or necrosis with neutrophils may be present (Fig. 38.9) and in some cases extensive. Fibrin thrombi may be seen in small veins. The widespread fibroblastic proliferation is often seen as a sign of “chronicity” by pathologists but is, in fact, one of the hallmarks of the acute cholecystitis syndrome. This “tissue culture–like” (myo)fibroblastic proliferation (Fig. 38.10) begins by the 5th day and peaks around the 10th. Along with this, lymphocytes, plasma cells, eosinophils, and pigment-laden macrophages appear. The histopathologic picture may be superimposed on prior chronic cholecystitis, further contributing to the discrepancy between the clinician’s opinion and the pathologist’s designation of the process as “acute” or “chronic.” The epithelium often exhibits reactive atypia (Fig. 38.11), which is severe in some cases and may be misinterpreted as dysplasia (see the dysplasia section for the differential from dysplasia). On occasion, fibrinoid necrosis of the muscular arteries may be seen. Some of these patients may later be diagnosed with a systemic collagen vascular disease and, therefore, this possibility may be noted.
Complications
If untreated, acute cholecystitis may lead to free perforation, which has now become an uncommon occurrence after the widespread application of cholecystectomy. Perforations may lead to fistula formation with neighboring organs. Rarely, bile may leak from the distended organ into the peritoneal cavity even in the absence of a visible perforation, leading to bile peritonitis, a condition often with a fatal outcome. Florid, acute cholecystitis may result in focal or diffuse mural infarction with subsequent perforation in a subset of these patients. The diagnosis of acute gangrenous cholecystitis is rendered for cases with true necrosis of the wall of the gallbladder but not for those with severe mucosal ulceration without mural infarction. Bacterial invasion may ensue in some patients with acute calculous cholecystitis as a secondary event. On the other hand, infection by gas-forming bacteria (most commonly Clostridium perfringens), especially in diabetics with acalculous cholecystitis, may be associated with mural gas gangrene and is radiologically identified by pneumobilia (acute gaseous cholecystitis or emphysematous cholecystitis) (86,87). Intramural air in this condition may be identifiable microscopically as spaces surrounded by inflammation that includes neutrophils, eosinophils, and giant cells (88).
Empyema (89), a pus-filled gallbladder with obstructed cystic duct, is a severe form of acute cholecystitis that is regarded by some separately from ordinary acute cholecystitis. The presentation is often atypical, and the diagnosis may be missed clinically. Often, the mucosa is extensively ulcerated and contains numerous neutrophils. The mortality rate is high (15% to 25%).
Clinical Course and Treatment
The treatment of acute cholecystitis is cholecystectomy (90). If untreated, localized perforation develops in 10% and peritonitis in 1% of cases. In the remaining untreated patients, the attack subsides in 7 to 10 days and often within 24 hours; however, recurrences are common. In approximately 25% of patients, the attack becomes progressively more severe, requiring immediate surgical intervention. The overall mortality rate for acute calculous cholecystitis is about 1%. Acute acalculous cholecystitis, however, has a more fulminant clinical course with a mortality rate approaching 40%, possibly related to the underlying conditions.
CHRONIC CHOLECYSTITIS
Epidemiology, Clinical Features, and Pathogenesis
Chronic cholecystitis (90,91) is the most commonly encountered disease of the gallbladder; the overwhelming majority of cholecystectomies are performed for chronic cholecystitis. It is associated with cholelithiasis in more than 90% of the cases. Therefore, as with gallstones, there is a female predominance. Although it may develop as a sequela of recurrent acute cholecystitis, many times there is no history of antecedent attacks. Some patients are discovered incidentally when gallstones are detected during workup for other disorders.
How gallstones lead to chronic cholecystitis is not well understood, and in fact, whether they play a direct role in the initiation of the inflammation or the development of pain is debated. There is poor correlation between the severity of the injury and the number and volume of the stones (92). It is speculated that intermittent obstruction and alteration in bile chemistry leads to mucosal irritation. In up to one-third of the patients, bile cultures are positive, usually for E. coli or enterococci. Recently, studies have found Helicobacter species; its possible role in the process is under scrutiny (93–96). Very rarely, Giardia lamblia may be present, especially in patients with IgA deficiency, achlorhydria, or malabsorption. Salmonella typhi may be present in the gallbladder in chronic carrier states.
Macroscopic Findings
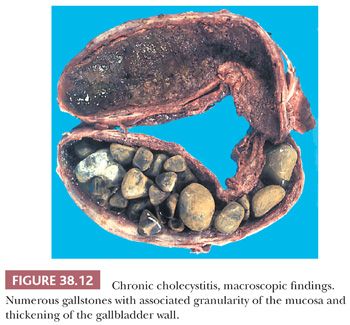
The pathologic findings vary depending on the severity and the duration of the disease. Calculi are present in greater than 90% of the cases (Fig. 38.12). The gallbladder itself may appear nearly normal or show thickening of the wall. In more severe cases, the gallbladder may be shrunken with marked fibrous thickening of the wall and serosal scarring associated with adhesions to adjacent organs. In addition, the mucosa may appear granular, ulcerated, or exhibit regenerative polypoid areas, which can be prominent in some cases. These small bumps usually display a mixture of cluster of pyloric glands and stroma, composed of myoid cells and fibrosis.
In about 1% of cholecystectomies, the gallbladder wall becomes a relatively thin, homogenous, and firm band of hyalinizing fibrosis that effaces the layers of the gallbladder. This is often but not always accompanied by calcifications of variable amounts. This process has been recently termed hyalinizing cholecystitis. Recent studies have shown that hyalinizing cholecystitis with minimal/no calcifications, in contrast to the diffusely calcific ones, have a close association with cancer (see the following texts for details) (97,98).
Microscopic Findings
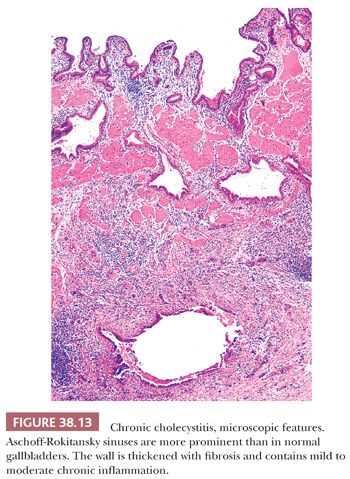
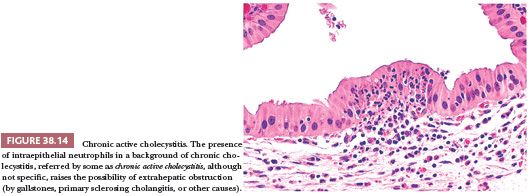
The degree of chronic inflammation varies and is composed predominantly of T lymphocytes along with some plasma cells, histiocytes, and occasional eosinophils. There is often thickening of gallbladder wall (Fig. 38.13). In many cases, however, the inflammation is so scanty that the distinction between normal gallbladder and minimal chronic cholecystitis is subjective. It should also be remembered that cholelithiasis may be symptomatic even in the absence of chronic cholecystitis. Some advocate rendering the diagnosis of chronic cholecystitis only when the gallbladder is obviously inflamed or showing signs of injury such as fibrosis or thickening. When neutrophils are also present (Fig. 38.14), the term chronic active cholecystitis may be preferable and obstruction should be suspected. In some cases, collections of macrophages that contain a mixture of bile and lipofuscin may be seen (cholegranulomatous cholecystitis).
Fibrosis is also variable. Obliterative changes in the lumen of small vessels and neural hyperplasia (in a traumatic neuroma–like pattern) may be seen. We have seen cases with neural proliferation in the lamina propria resembling the mucosal neuromas (ganglioneuromas) of the GI tract. There may be elastotic changes in the perimuscular tissue.
The epithelium may be atrophic, hyperplastic, normal, focally ulcerated, metaplastic, or regenerative (99). Metaplastic changes (99,100) of pyloric gland type are common (see normal histology in earlier discussion and metaplastic changes in the following section); these are sometimes the main evidence of chronic cholecystitis.
Diverticular changes known as Aschoff-Rokitansky sinuses (10) are very common in chronic cholecystitis and are more prominent in more severe cases (Fig. 38.13). Strictly speaking, these are pseudodiverticula, composed of deeply invaginating mucosa without a complete muscular coat. They form irregularly shaped tubular structures in the wall of the gallbladder and may contain bile or calculi. In chronic cholecystitis, these may display reactive epithelial atypia, which may be quite severe, and mistaken for invasive carcinoma. On the other hand, many gallbladder carcinomas arise in conjunction with cholelithiasis and cholecystitis and may appear deceptively benign. It may prove quite challenging to distinguish these sinuses from invasive carcinoma (see discussion on carcinoma).
Hourglass gallbladder may develop as a complication of chronic cholecystitis; the septum is composed of inflamed fibrous tissue or adenomyomatous hyperplasia. Whether adenomyomatous nodules discussed separately later are an exaggerated form of Aschoff-Rokitansky sinuses, and as such, an evidence of chronic injury, or a developmental lesion has yet to be determined (101,102).
VARIANTS OF CHRONIC CHOLECYSTITIS
Hyalinizing Cholecystitis (Porcelain Gallbladder)
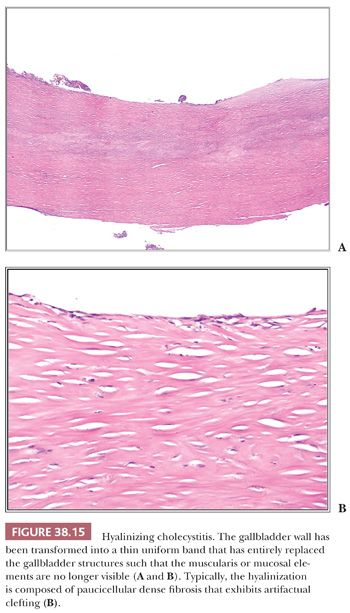
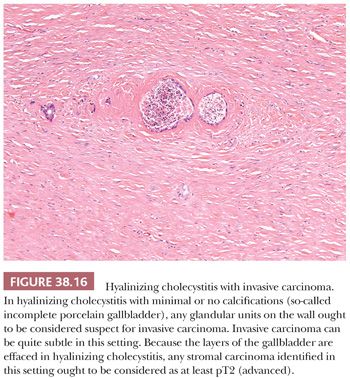
In 1% to 2% of cholecystectomies, the gallbladder wall is transformed into a uniform, thin, densely hyalinized band effacing the normal gallbladder structures (Fig. 38.15). This is seen in patients about a decade older than ordinary cholecystitis, suggesting that it may be a long-term sequelae of chronic injury that ensues in a certain subset of the patients. Microscopic examination reveals a paucicellular hyaline fibrosis in place of the layers of the normal gallbladder; in established cases, neither the muscularis nor any epithelial elements remain. In fact, in this setting of dense hyaline fibrosis, any glandular elements ought to be considered suspect for carcinoma (Fig. 38.16). This dense hyaline fibrous tissue often exhibits a characteristic artifactual clefting. Calcifications are often present but not always identifiable. Porcelain gallbladder is the term that had been used in the old literature for those that are diffusely calcific, but recent studies (23,103) have shown that it is actually not the diffusely calcific ones (97,98) but rather the established hyalinizing cholecystitis (with total or almost total effacement of the layers) with minimal or no calcifications (“incomplete porcelain”) that confer the well-known high cancer risk to these lesions (23,97,98,103,104). The carcinomas that arise in hyalinizing cholecystitis are often difficult to diagnose because they can be fairly subtle. Clear cell cytology, nuclear enlargement, and axis parallel to the surface are the main features that help recognize their malignant nature. They are also difficult to stage properly because the layers of the gallbladder are no longer evident; any invasive carcinoma identified in this setting ought to be regarded as at least pT2 (advanced).
Mucosal-Predominant Lymphoplasmacytic Cholecystitis
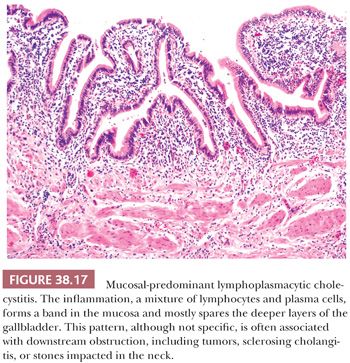
A form of chronic acalculous cholecystitis characterized by a diffuse plasma cell–rich inflammatory infiltrate mostly confined to lamina propria (not extending to deeper layers) (Fig. 38.17) and that involves the mucosa rather diffusely is a distinctive form of cholecystitis originally thought to be associated with autoimmune disorders such as ulcerative colitis (105), primary sclerosing cholangitis (106), and autoimmune pancreatitis (107–110). Later, in some studies, this phenomenon was considered to be part of the IgG4-related autoimmune disorders that afflict the pancreatobiliary tract and accordingly was termed sclerosing cholecystitis (107,108). More recently, however, it is becoming clear that mucosal-predominant chronic inflammation may in fact be a sign of downstream obstructive processes (110,111), autoimmune or otherwise, rather than being a specific injury pattern of autoimmune attack of the gallbladder. In other words, this pattern is seen more commonly in cases with obstructive processes including distal biliary and pancreatic/ampullary tumors, choledocholithiasis, impacted stones in the cystic duct, in addition to immune-based sclero-inflammatory conditions of the biliary system. In the cases of primary sclerosing cholangitis, the surface epithelium tends to become attenuated and in some cases is also associated with peribiliary mucous gland hyperplasia (Fig. 38.3). In IgG4-related cases (see the following texts), the distinctive fibroplasia and intraepithelial neutrophils can be noted. In those due to obstruction, the mucosa tends to be more hyperplastic with vascular ectasia in the tips of the lamina propria of the hyperplastic mucosa. However, none of these findings and patterns appear to be specific for any of the etiologies (111).
IgG4-Related Sclerosing Cholecystitis
Gallbladders removed along with the pancreatoduodenectomy specimens in patients with IgG4-related (lymphoplasmacytic) sclerosing pancreatitis (autoimmune pancreatitis type 1) often show inflammatory changes in the gallbladder as well (112–117). Commonly, this inflammation is mostly mucosal-predominant lymphoplasmacytic type discussed earlier (111), which is often attributable to the obstructive changes in the distal CBD that is common in this disorder (118–120). In some cases, however, morphologic findings similar to that occur in the IgG4-related autoimmune pancreatitis are seen. These include distinctive basophilic wavy sclerosis forming a band around epithelial units, with plasma cells lying individually in lacunae created by this sclerosis (112,121). Rarely, this can be discovered independently in a cholecystectomy specimen (112), unaccompanied by pancreatic involvement and obstruction. In such cases, a more specific diagnosis of IgG4-related sclerosing cholecystitis may be justifiable. However, a purist’s approach is required, with the presence of all the morphologic characteristics as well as careful clinical correlation with exclusion of other etiologies before such a diagnosis can be rendered. It should also be kept in mind that IgG4-positive plasma cells are not specific for any condition and can be prominent in a variety of injury patterns, (122) including ordinary gallstone-associated cholecystitis (112).
AIDS-Related Cholecystitis
Acalculous cholecystitis is frequently encountered in patients with AIDS (123). More than half of these are idiopathic; however, in the remainder, specific infectious agents, in particular, cryptosporidia and CMV may be detected (124). As in the GI tract, CMV may be associated with ulceration, whereas cryptosporidia cause little inflammation. Other organisms such as Candida, Mycobacterium, and microsporidia (Enterocytozoon bieneusi and Encephalitozoon intestinalis) may also be seen (125).
Follicular Cholecystitis
Lymphocytic infiltration is a part of chronic cholecystitis, and scattered follicles may be encountered in ordinary chronic cholecystitis. Cases in which numerous prominent lymphoid follicles (defined in some studies as three follicles per centimeter) are present in the lamina propria throughout the gallbladder (Fig. 38.18), creating a pseudolymphoma pattern (126), are referred to as follicular cholecystitis (127–129). Follicles can be present on the wall as well but are typically predominantly mucosal. In some cases, the follicles form a compact lymph node–like structure that surrounds the Aschoff-Rokitansky sinuses. Follicular cholecystitis constitutes less than 2% of cholecystectomies. Patients are typically in their mid-60s, significantly older than ordinary cholecystitis cases. It does not seem to have any specific association with obstructive or immune-based injury (129). Originally described in typhoid fever, it is now known to occur in other conditions such as Gram-negative infections (130) and others. In a recent study, a third of the patients were found to have biopsy-proven gastritis (129).
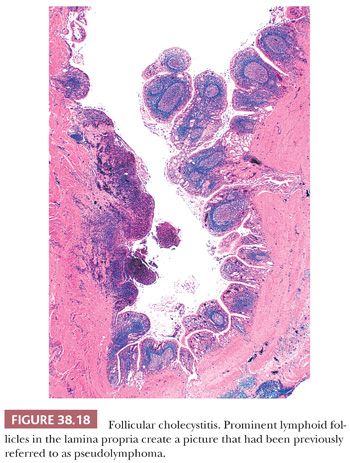
Eosinophilic Cholecystitis
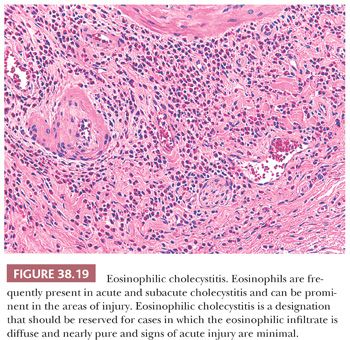
Approximately 20% of resected gallbladders have eosinophils, and a prominence of eosinophils in an otherwise polymorphous infiltrate is a relatively common finding in subacute cholecystitis and to a lesser degree, in acute cholecystitis (131). Eosinophils tend to be more prominent in areas of ulceration, leading to the speculation that their presence may be a reaction to the chemical injury by bilious contents. In contrast, in chronic cholecystitis without a breach of mucosal integrity, predominance of eosinophils (Fig. 38.19) is very uncommon (<0.7%) (131–136). When defined stringently by almost exclusive predominance of eosinophils as the primary inflammatory cells and by the absence of ulceration, eosinophilic cholecystitis is a rare process that seems to be more common in younger females with allergies (131). Some examples of eosinophilic cholecystitis are reported in association with eosinophilic gastroenteritides (133). Parasitic diseases of the liver and biliary tract may also be associated with eosinophilic cholecystitis. Drug hypersensitivity has also been implicated. In many cases, however, it is an isolated phenomenon with no associated disorders. Gallbladder involvement by granulomatous angiitis with eosinophilia (Churg-Strauss syndrome) should be distinguished from eosinophilic cholecystitis where vasculitis is absent.
Xanthogranulomatous Cholecystitis
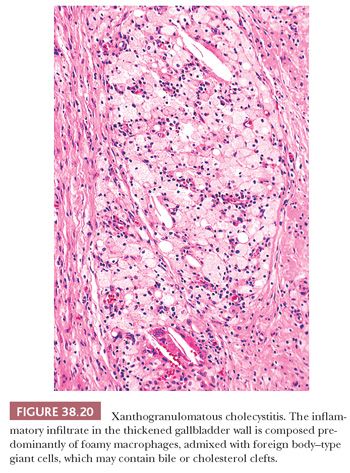
Xanthogranulomatous cholecystitis (137–139) is characterized by a prominent proliferation of foamy macrophages (Fig. 38.20), often associated with cholelithiasis. It is speculated that ulceration of the gallbladder mucosa and/or rupture of Rokitansky-Aschoff sinuses with extravasation of bile is the inciting event (140). Some examples display plaque-like thickening of the gallbladder, whereas others are more nodular, forming grossly and radiologically detectable masses (“pseudotumors”) (140) and occasionally even polypoid nodules. Grossly, the process has a well-delineated yellow color. In addition to cholesterol, foam cells also often contain pigment (bile, ceroid, and less frequently, iron). Other inflammatory cells including lymphocytes, plasma cells, and Touton-like or foreign body–type giant cells (often with cholesterol crystals) are usually admixed with the foamy macrophages. In the organization stage, fibroblastic proliferation may predominate. Rare cases with Michaelis-Gutmann bodies have been described, justifying the diagnosis of malakoplakia (141,142). A highly debatable association with gallbladder carcinoma has been reported (140,143). Some patients were also reported to have high serum CA19-9 level in the absence of any identifiable carcinoma (144).
OTHER INFLAMMATORY CONDITIONS
Infections
Most infections of the gallbladder occur as a secondary phenomenon superimposed on an underlying pathology such as gallstones or neoplasia and present with the clinicopathologic picture of chronic cholecystitis (67). Gut flora, in particular E. coli, is the most common source. Some bacteria, however, have an affinity for the gallbladder as one of the primary sites of disease. For instance, in Salmonella infections, the gallbladder serves as a reservoir for the microorganisms during the course of typhoid fever, as well as in the “carrier” state, although they may not cause any pathology in the gallbladder itself. Some studies have found a higher prevalence of Salmonella positivity in patients with gallbladder cancer. Also, in cholera, the gallbladder is one of the sites involved primarily by the microorganism. In addition, a variety of other infectious agents may affect the gallbladder, including opportunistic microorganisms (see the section “AIDS-Related Cholecystitis”), parasites (such as giardiasis, schistosomiasis, and amebiasis) (145), and viruses (in particular, EBV, hepatitis A, and CMV).
Vasculitis
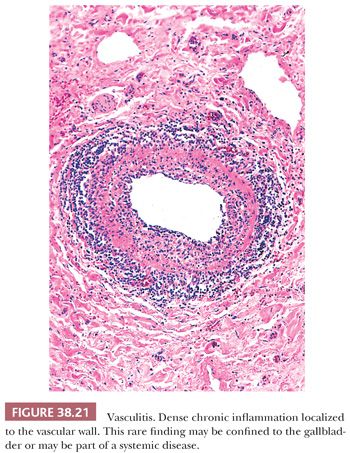
Vasculitis (Fig. 38.21) may be encountered incidentally in gallbladders removed for cholelithiasis (146–148). It may also be found in patients that present clinically as acalculous cholecystitis. It is characterized by preferential, full-thickness inflammation of the vessels, in the absence of an inflammation in the perivascular soft tissues. Fibrinoid necrosis may or may not be present. Vasculitis should be distinguished from secondary, bystander inflammation of the vessels in the setting of diffuse inflammation and should be duly noted because it may be a manifestation of a systemic disease. Although rare, a variety of collagen vascular disorders including rheumatoid arthritis, systemic lupus erythematosus, Henoch-Schönlein purpura, mixed connective tissue disease, and Wegener granulomatosis may involve the gallbladder. However, vasculitis in gallbladder is more likely to be a localized phenomenon, and some consider this as a monoarterial form of polyarteritis nodosa (149). Vasculitis has also been reported in patients with intrahepatic arterial chemotherapy.
ISCHEMIA AND INFARCTION
Although most gallbladder infarctions are related to cholecystitis (see earlier discussion), it may also seldom develop as an acute event attributable to ischemia related to thromboembolism, torsion (volvulus) of the gallbladder, surgical interventions, penetrating duodenal ulcer, cocaine abuse, or even hypertension without vascular occlusion. Depending on the cause and severity of the insult, the infarction may be more localized or diffuse. Those associated with a thickened gallbladder wall frequently contain calculi. Microscopically, the epithelium is denuded and may be replaced by granulation tissue. Chronic ischemic changes akin to those in the GI tract have not been described in the gallbladder.
NONNEOPLASTIC EPITHELIAL ALTERATIONS
Hyperplasia
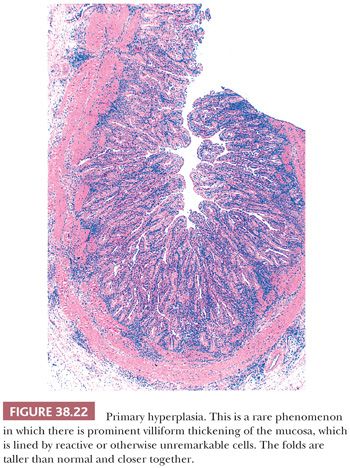
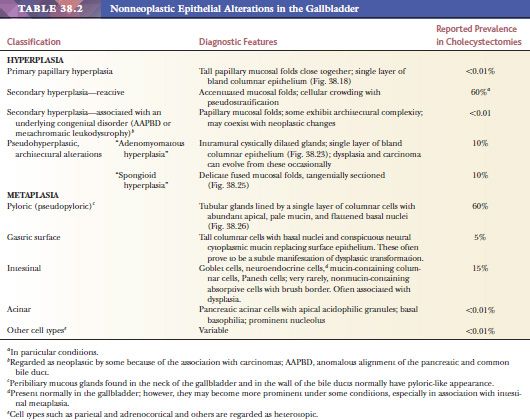
In hyperplasia, a relatively normal-appearing epithelium forms more prominent folds than would be expected in the normal gallbladder. Some degree of microscopic accentuation or crowding of mucosal folds in the gallbladder mucosa is not uncommon and may be seen as a reaction to injury, incited usually by gallstones or cholesterolosis. It can be quite striking and accompanied with edema of the cores, in particular, in cases with downstream obstruction. Primary papillary hyperplasia, if defined strictly, is an exceedingly uncommon phenomenon to an extent that its existence is debatable. It is characterized by a thickened gallbladder mucosa which exhibits villous-like folds that are taller than normal and closer together (Fig. 38.22), without any distinct nodule formation (150,151). The epithelium appears normal, and no Paneth cells, endocrine cells, or metaplastic goblet cells are seen. The process may be focal, segmental, or diffuse (152).
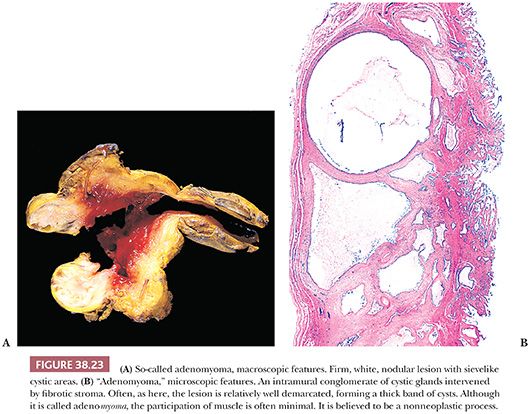
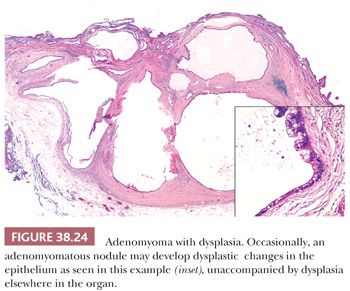
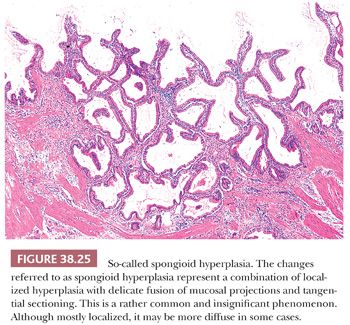
The process referred as adenomyomatous hyperplasia is technically not a hyperplasia but rather an exaggerated form of diverticular changes, or a malformation, and does not involve epithelial hyperplasia (see the following discussion; Fig. 38.23). It can, however, occasionally develop dysplasia (Fig. 38.24). So-called spongioid hyperplasia (8) probably represents a combination of localized hyperplasia and tangential sectioning that imparts a complex appearance (Fig. 38.25). The epithelium is otherwise unremarkable.
The atypical papillary epithelial proliferations noted in the gallbladders of patients with “anomalous arrangement of the pancreatobiliary ducts” (adjoining of the CBD and pancreatic duct outside the duodenal wall) are likely early forms of neoplastic transformation (153) and should probably be classified as such (dysplasia). Along the same lines, it is not clear whether the papillary proliferations seen commonly in the gallbladder of children with metachromatic leukodystrophy (154,155), also referred to as sulfatide cholecystosis, represent primary or secondary hyperplasia or dysplasia.
Metaplasia
Metaplastic changes (100,156–158), usually of gastric or intestinal types and rarely of others such as squamous and pancreatic acinar types, are commonly associated with gallstones and increase with age (158). These changes may be seen in any region of the gallbladder and can be focal or diffuse.
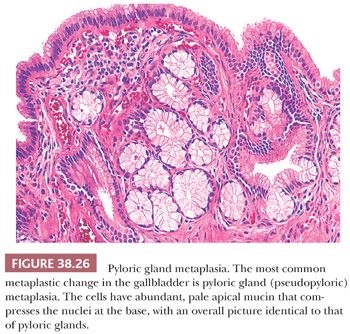
Pyloric metaplasia (also called pseudopyloric, antral, or mucous gland metaplasia) is the most common type of metaplastic change in the gallbladder, found in more than 75% of cholecystectomy specimens (Fig. 38.26). Collections of metaplastic glands may be present in the lamina propria and occasionally also involve the deeper aspects of the muscular layer (Fig. 38.27). In the mucosa, they may form microscopic polyps and nodules. In fact, most of the cases reported in the literature as “pyloric gland adenoma” were subcentimeter lesions, which we believe represent exaggerated forms of these polypoid metaplasias. We prefer not to designate such polyps as neoplastic (or adenoma) unless they have clear-cut cytologic dysplasia or form a distinct tumor greater than 1 cm. The glands indeed resemble gastric pyloric glands and differ from the mucous glands found in the gallbladder neck microscopically, histochemically, and ultrastructurally. In addition to neutral mucins typical of pyloric glands, however, they may also contain small amounts of sialo- and sulfomucins. In other words, they may not be perfect examples of pyloric glands and that is why some authors refer to these as “pseudopyloric.” In some cases, immunohistochemical expression of estrogen receptors has been detected in these glands. The presence of chief and parietal cells is a feature regarded as indicative of heterotopia rather than metaplasia, and this is quite uncommon.
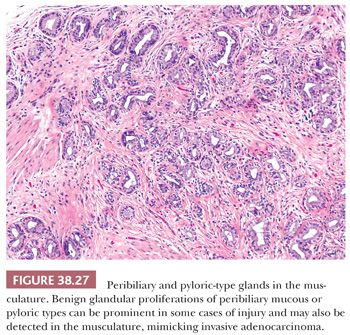
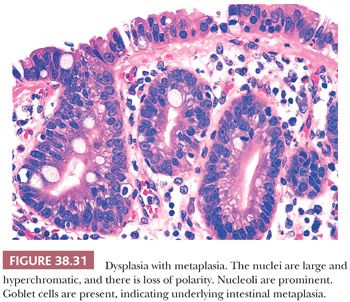
Intestinal metaplasia, characterized by the presence of goblet cells (that contain sialomucin), is much less common in cholecystectomy specimens than pyloric gland metaplasia. This typically appears to be “incomplete” metaplasia because the background cells rarely show a distinct brush border. Paneth cells may be present occasionally. As in the stomach, intestinal metaplasia is believed to have an association with carcinoma. The incidence of intestinal metaplasia is significantly higher in gallbladders with dysplasia or carcinoma as well as in gallbladders from high cancer-risk regions (159), further highlighting its association with carcinoma. Additionally, goblet-like cells often occur within the dysplastic epithelium and are fairly uncommon in nonneoplastic cases. For this reason, further scrutiny of the mucosa is recommended, typically with submission of two additional blocks with multiple fragments in each, if goblet-like cells or intestinal metaplasia associated with atypia is identified. On the other hand, there is emerging evidence that the presence of goblet cells may be a marker of more indolent behavior in gallbladder carcinogenesis.
Neuroendocrine cells can be highlighted by immunohistochemical stains in 15% of cholecystectomy specimens (157,160). Most are serotonin-containing, and the remainder express somatostatin, cholecystokinin, gastrin, or pancreatic polypeptide. As in the GI tract, neuroendocrine granules can be distinguished from Paneth cell granules by their finer appearance; more dull, amphophilic color; and localization predominantly in the infranuclear aspect of the cytoplasm.
Squamous metaplasia is an extremely uncommon event in the gallbladder. It has been noted in “porcelain gallbladder” (see earlier discussion) and also adjacent to squamous cell carcinomas of this organ.
Pancreatic acinar cells can be encountered occasionally in gallbladders. It is debated whether these are heterotopic or metaplastic. Our impression, based on their distribution, size, association with other metaplastic processes (especially pyloric gland type), and absence of other pancreatic elements, is that they most likely represent metaplasia rather than heterotopia.
See Table 38.2 for relative frequencies of the aforementioned processes and a summary of their diagnostic features.
NONNEOPLASTIC TUMORLIKE LESIONS
There are a variety of inflammatory, reactive, regenerative, and congenital processes in the gallbladder that may form small tumorlike lesions (Table 38.3). Advances in imaging technology have led to the increased detection of these small (most measure less than a centimeter) and often inconsequential lesions (151,161–164), many of which are relatively easy to recognize by their context.
Polyps
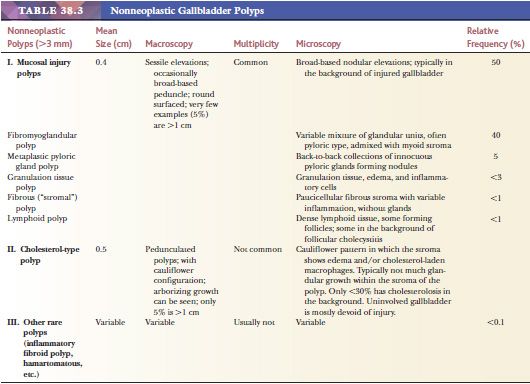
I. Mucosal Injury Polyps
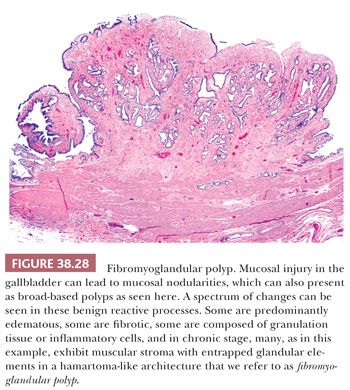
FIBROMYOGLANDULAR POLYPS. Many patients with chronic cholecystitis develop significant mucosal nodularity and develop into broad-based polyps distinct from the remainder of the mucosa. Microscopically, these are typically composed of fibrosis with variable myoid component admixed with glandular elements, and for this reason, we prefer to classify these as fibromyoglandular polyps (165) (Fig. 38.28). Depending on the predominant pattern, they have also been termed in the past as fibroepithelial or fibroinflammatory polyps (166) and those with edematous or fibrous stroma and few scattered inflammatory cells as fibrous polyps (Fig. 38.28). However, in most cases, the fundamental process includes fibromyxoid stroma admixed with variably distributed glandular elements. In many respects, these are akin to prolapse-related polyps seen in the GI tract. They occur in patients who are almost a decade older than ordinary cholecystitis patients, also suggesting that they are long-standing changes due to injury. They are typically multiple and small; most are less than 0.5 cm, and only less than 5% are greater than 1 cm (167). Some of these appear to be healed granulation tissue polyps, whereas others have more prominent pyloric gland participation and transition into the polypoid pyloric gland metaplasias discussed in the following section. The surface epithelium may be denuded but in some cases show regenerative hyperplasia. About 15% harbor dysplastic changes but this is typically as an innocent bystander, in the context of dysplasia and carcinoma involving the rest of the mucosa.
POLYPOID PYLORIC GLAND METAPLASIA. Pyloric gland metaplasia is a common finding in chronic injury of the gallbladder, and it often forms polypoid collections (Fig. 38.26). Some of these have been dignified with the diagnosis of pyloric gland adenoma. In fact, in the literature, the average size of the lesions in the largest series on so-called pyloric gland adenomas was 0.7 to 0.9 cm (168–170). However, we prefer not to classify these as neoplastic (adenoma or otherwise) unless they show overt cytologic dysplasia or form compact nodules that are distinct from the neighboring mucosa (171). In order to classify a pyloric gland nodule as “neoplastic” (see later discussion on intracholecystic papillary tubular neoplasms), we use the cholecystectomy size criterion of 1 cm, which is also the size criterion used to distinguish mass-forming preinvasive (tumoral intraepithelial neoplasms) from lesser lesions in the pancreas and biliary tract (171–173).
INFLAMMATORY POLYPS. A variety of inflammatory changes lead to polypoid arrangement in the gallbladder (165). Granulation tissue polyp and broad-based mucosal nodules of xanthogranulomatous cholecystitis are the two best examples, both of which typically occur in the setting of severe cholecystitis, acute, or subacute. Lymphoid polyps are pedunculated or sessile protrusions containing lymphoid follicles covered by epithelium. They may be single or multiple and typically arise in chronic cholecystitis, in particular, follicular cholecystitis discussed earlier.
STROMAL POLYPS. Depending on the phase of the injury, some mucosal polyps of the gallbladder are composed almost exclusively of stromal elements (165). “Fibrous polyps,” as discussed in “Fibromyoglandular Polyps” section earlier, can be almost entirely fibrotic. Another, what seems to be an injury-related process of uncertain etiology, is formed by the clusters of nerve sheath cells akin to the Schwann cell hamartomas described in the GI tract (174,175). These appear to occur more commonly in cases with cholesterolosis. Some cases have been designated “neurofibroma-like” polyps.
II. Cholesterol Polyps. Among the polyps occurring in uninjured gallbladders, cholesterol polyps are by far the most common (151,165,176). More than a third of the patients are male. In many ways, they are regarded as a polypoid version of cholesterolosis in which lipid-laden macrophages fill the lamina propria underlying gallbladder epithelium. However, in a significant proportion of cases, there is no cholesterolosis in the uninvolved mucosa; and furthermore, in many cases, stromal macrophages are minimal, and in 14%, they are undetectable (165). Instead, the stroma may show edema, which is presumably lysed product of the macrophages. The architecture is very distinctive cauliflower-like and pedunculated, typically with a thin stalk (Fig. 38.29). This characteristic architecture allows its recognition as a cholesterol polyp even in the absence of cholesterol macrophages. Some are multiple but are usually not associated with diffuse cholesterolosis. Only a small percentage occurs with cholelithiasis. Also, patients with cholesterol polyps seldom show findings of chronic cholecystitis such as mural thickening.
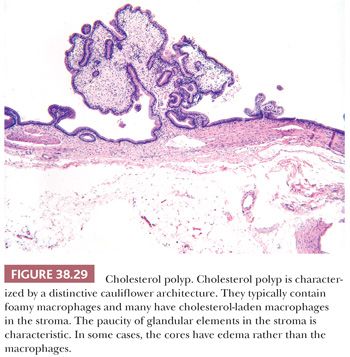
Cholesterol polyps are generally believed to be innocuous processes; however, recent studies elucidated the presence of dysplasia in a small percentage of cases (4%), mostly low grade (177). Additionally, the distinctive subset of the intracholecystic papillary neoplasms referred as complex-tubular type may arise from cholesterol polyps, as evidenced by architecture similar to that of cholesterol polyps as well as the presence of cholesterolosis within the polyp in 35% of the cases (178). However, these are virtually never associated with invasive carcinoma.
III. Other Rare Specific Polyp Types. Inflammatory fibroid polyp is very uncommon in the gallbladder (179,180). The morphologic features appear to be fairly similar to those in the GI tract. One example we have seen showed more neural-like features.
Hamartomatous polyps composed of disorganized and focally cystic epithelial elements have been reported in the gallbladder in patients with Peutz-Jeghers syndrome and Cowden disease (7,181).
Heterotopias may present as nodules or polyps as well. They may measure up to 2 cm. Gastric heterotopia may lead to a cholecystitis-like clinical picture in young adults or children and may be associated with ulcers (182).
Cysts
Adenomyomatous nodules (see the following section) and Aschoff-Rokitansky sinuses (diverticular changes) may sometimes lead to small cysts or a sievelike cystic trabeculation of the gallbladder wall. Luschka ducts (see “Histology” section) may also form small, usually microscopic, cysts. The recently recognized tubulocystic variant of biliary adenocarcinoma (183) may present a deceptively benign-appearing cystic lesion in the gallbladder wall.
Adenomyomatous Nodules
“Adenomyoma,” also called adenomyomatous hyperplasia, is the name given to a mural collection of cystically dilated glands forming a small solitary mass or a band of trabeculated thickening of the gallbladder wall with sievelike configuration (Fig. 38.23), most often in the fundic region (184). The process is not neoplastic but rather appears to be developmental. Some regard it as an exaggerated form of Rokitansky-Aschoff sinuses, but in many cases, there is no evidence of injury, no other sinuses elsewhere, no overt communication with the surface mucosa, and the configuration of the glands are not typical for Rokitansky-Aschoff sinuses. Additionally, although the name indicates a myoid process, the muscular component is often minimal or lacking. The process may give rise to polypoid lesions as well. “Adenomyomatosis” refers to the more diffuse form of this condition, which is very uncommon.
In addition to mimicking adenocarcinoma themselves due to their pseudoinvasive appearance, adenomyomas may also develop in situ or invasive carcinoma (185) (Fig. 38.24), and we have seen several examples of this phenomenon, in which the only in situ and invasive carcinoma was occurring in and in the vicinity of the adenomyomatous nodule. It should be noted that some glandular elements in benign adenomyomatous nodules may impinge on the nerves, mimicking perineurial invasion (186,187). In some cases, adenomyomas have diffuse dysplastic change accompanied by papillary configuration, creating a picture that has been referred as mural intraductal papillary mucinous neoplasm (188).
Inflammatory Pseudotumors
Some inflammatory conditions of the biliary tree may occasionally be associated with an exuberant (myo)fibroblastic proliferation that may be clinically mistaken for carcinoma (189). Xanthogranulomatous cholecystitis is prone to lead to this picture (138,190). Eosinophilic cholecystitis may also be associated with pseudotumor formation, especially in the extrahepatic biliary tree (191), and these may also be associated with pseudotumor-like changes in the gallbladder as well. Some of these may be associated with (or an extension of) IgG4-related sclerosing disorders (192).
Amyloidosis
Rarely, amyloidosis involves the gallbladder (193), often as a part of a systemic process. We have seen an example in which amyloid was extensively deposited in the muscularis in addition to the vessel walls.
NEOPLASIA OF THE GALLBLADDER
Preinvasive Epithelial Neoplasia
General Considerations. As in other epithelial organs, there are various types and grades of noninvasive (preinvasive) neoplasia in the gallbladder. They can be separated into two categories (171,194,195). The first consists of the microscopic (nontumoral) intraepithelial neoplasia (dysplasia) that can be found in gallbladders involved by invasive adenocarcinoma and, on occasion, in cholecystectomies performed for other reasons. The second group is the mass-forming type of preinvasive neoplasia, representing the “adenoma–carcinoma” sequence and reflected in the categories designated “adenoma” and “intracystic papillary neoplasm” by the World Health Organization (WHO) 2010 (187), which we refer to collectively as intracholecystic papillary tubular neoplasm (ICPTN) (171). These two groups will be discussed separately in the following texts, but there are some common issues pertinent to both groups that will be addressed first:
1. Biologic significance and risk. That these preinvasive lesions are precursors, and even “markers” of invasive carcinoma (developing elsewhere in the biliary tree), is widely accepted based on (a) their close association with invasive carcinoma, both spatially and epidemiologically (196–202); (b) their expression of cytomorphologic indicators of neoplastic transformation; and (c) analogy with similar but better established examples in other organs. However, their absolute and relative risk of progressing into something that requires therapeutic intervention needs to be further analyzed. It is particularly problematic when they are detected in the absence of invasion or at the margin of a resection; clinical follow-up of the biliary tree with radiologic, serologic, and other methods is inadequate, complex, and difficult. In cases with high-grade dysplasia/carcinoma in situ (CIS) in which the possibility of invasion into perimuscular tissue has been ruled out with complete sampling, the prognosis appears to be very good, with 10-year survival about 90% (16,203–205). Rokitansky-Aschoff involvement has been found to be an adverse prognosticator in these cases. Some of the recurrences/metastases take place long after cholecystectomy (8 to 10 years later), leading to the conclusion that they may represent new biliary tract cancers, as a result of a field effect phenomenon (“marker” disease).
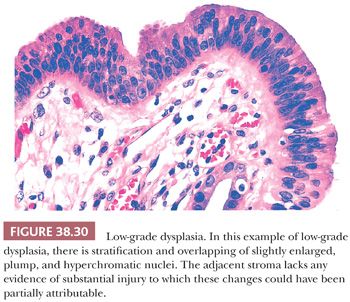
2. Grading. In the gallbladder, based on the degree of cytologic and architectural atypia, dysplasia is generally graded as low or high grade (Figs. 38.30 to 38.32). Unfortunately, this can be highly subjective. Although the criteria used are similar to those applied in the GI tract (see intraepithelial neoplasia in the following section), some subtle differences do exist as will be discussed later.
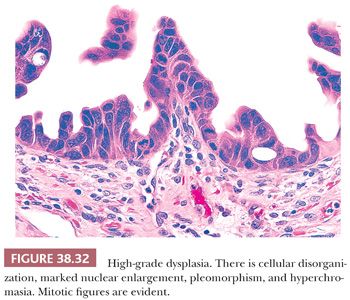
3. Distinction from reactive atypia. Injury to gallbladder and biliary tree may incite tremendous cytomorphologic atypia in the epithelial cells (Fig. 38.11). The distinguishing features may be subtle or downright nonexistent. Considering inflammation–carcinoma relationship is very striking in this organ (carcinoma may be a result of regeneration that has gone wrong), it may not be surprising that it is often difficult to know where regeneration ends and the neoplasm begins. As in other organs, if the epithelium is acutely inflamed or ulcerated, the findings in that area ought to be evaluated conservatively. Additional sampling of the tissue would be imperative. For focal epithelial atypia with the differential diagnosis of reactive versus low-grade dysplasia, obtaining two to four additional blocks is advocated (20,22). Immunohistochemical expression of p53, if present, favors the diagnosis of a dysplastic or malignant process; however, this is not absolute, and hence, individual cases should not be diagnosed solely on the basis of a p53 stain (206). The same is also true for Ki-67 index, which seems to be higher in dysplasia, but the overlaps are too wide, disallowing its utility in individual cases. In fact, in our experience, areas of reparative atypia often show brisk mitotic activity.
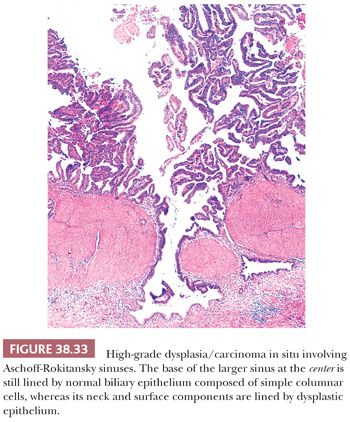
4. Distinction from invasive carcinoma. Dysplasia involving hyperplastic mucosal glands and showing pagetoid extension into Rokitansky-Aschoff sinuses (Fig. 38.33) is often difficult, if not impossible, to distinguish from invasion (197,202,207,208). Invasive carcinomas often show a different morphology than the surface noninvasive component, and they acquire a lower cuboidal appearance. Invasive tubules are relatively small, show either a dilated lumina or contour irregularities, and are more commonly lined by one or two cell layers of more cuboidal cells that show relative acidophilia. Noninvasive glands situated deep in the tunica muscularis often but not always have an even band of stromal tissue separating them from the neighboring muscle fibers. Of note, Rokitansky-Aschoff sinuses often have a concentric loose stroma around them mimicking desmoplasia.
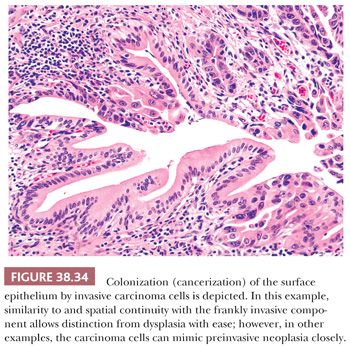
5. Distinction from colonization of surface epithelium (pagetoid spread) by invasive carcinoma. Invasive carcinomas may grow into the surface epithelium and it may be difficult, if not impossible, to tell this apart from CIS. Direct continuity and cytologic similarities of the two components favor “cancerization” (also known as colonization) (Fig. 38.34).
6. Distinction of the lower end of the spectrum from hyperplasia or metaplasia. It is difficult to identify the earliest signs of neoplastic transformation that take place. In addition, it seems that hyperplasia and metaplasia form the background in which neoplasias develop (Fig. 38.31) (159,209), often harboring common molecular alterations. As such, they can be regarded as the precursors of precursors (210). For nodular/polypoid versions of metaplasia without any overt cytologic atypia, we apply macroscopic or clinical detectability (thus the arbitrary criteria of 1 cm) to distinguish them from well-developed neoplasia (see the following texts) (171).
7. Dysplasia versus in-situ carcinoma. In the WHO 2010 (211) classification, the term carcinoma in situ has been largely eliminated from the GI tract pathology practice, to be replaced by high-grade dysplasia. It is not clear, however, how this will impact the staging and data collection because this makes the “Tis” category vaguer. In full-blown cases of CIS, we still prefer to employ the term high-grade dysplasia/carcinoma in situ.
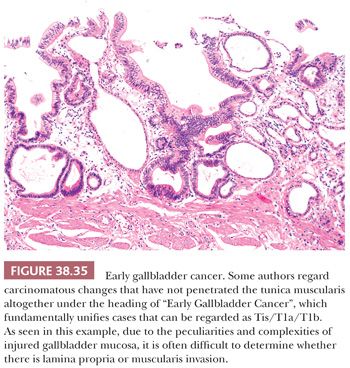
8. Tis versus “intramucosal carcinoma” and versus T1a versus T1b. In cases of injury, the gallbladder mucosa often shows striking complexity with hyperplasia and metaplasia of the glands, which often appear even within the tunica muscularis. When dysplastic processes involves these complex glands, it becomes very difficult to distinguish an “intramucosal adenocarcinoma” from mere dysplasia. Furthermore, the gallbladder does not have any muscularis mucosae, and the tunica muscularis is highly irregular and porous allowing numerous invaginations into and through (Fig. 38.35). This makes the application of the tumor-node-metastasis (TNM) classification adapted from the GI tract (with different layers) more or less inapplicable. It is often difficult, if not impossible, to distinguish Tis from T1a (lamina propria invasive) and even T1b (muscle invasive) in the gallbladder (Fig. 38.35). For this reason, authors from high-incidence regions developed the “Early Gallbladder Cancer” category for the muscle-confined cases, and they have demonstrated that their survival rate is incomparably better than more advanced carcinomas (perimuscular invasion; pT2) if thorough sampling is used (16). Therefore, in cases with uncertain or minimal invasion confined to the musculature, we refer to this early gallbladder cancer data.
I. DYSPLASIA AND CARCINOMA IN SITU, FLAT (NONTUMORAL) TYPE
For microscopic, incidental forms of preinvasive epithelial proliferations, the preferred terms, which are also endorsed by the WHO, are dysplasia or biliary intraepithelial neoplasia (BilIN) (187). In general, dysplasia is still more widely used. CIS had traditionally been regarded as the most severe form of dysplasia; however, WHO 2010 recommends avoidance of the term carcinoma in situ in the GI tract. This approach does not seem to have found wide acceptance in reference to the gallbladder, and we continue to report full-blown examples of dysplasia as high-grade dysplasia/CIS. In fact, because it is often difficult to distinguish these from early invasion (T1a/T1b), these cases are often included in the category of “Early Gallbladder Cancer” by authors in high-risk regions in Asia and South America (16).
Dysplasia is reported in 40% to 60% of the patients with invasive adenocarcinoma, and even these values probably represent an underestimation. On the other hand, the incidence of dysplasia outside the setting of invasive adenocarcinoma is difficult to determine, partly due to the subjectivity of the criteria. It is also clear that the frequency varies significantly between different populations and parallels that of adenocarcinoma. High-grade dysplasia/CIS is reported in 1% to 3.5% of cholecystectomies performed for gallstones and “low-grade” in up to 15% in areas of the world with a high incidence of gallbladder cancer (169,200,212–216). In contrast, in an analysis of routine cholecystectomies performed for gallstones and morbid obesity in a North American population, the incidence of low-grade dysplasia was less than 5%, high-grade less than 1%, and frank CIS less than 0.1% (217). The frequency of gallbladder dysplasia is also high in clinical conditions associated with a higher risk of biliary cancers such as primary sclerosing cholangitis (218–220), choledochal cyst, and anomalous union of pancreatobiliary ducts (221,222). Also, in our experience, there is a progressive increase in the mean age of patients with increasing degree of neoplastic transformation, from 50 years for low-grade dysplasia, 57 years for high-grade dysplasia without invasion, and 67 years for high-grade dysplasia associated with invasion (223).
Dysplasia that forms compact, grossly identifiable masses is discussed separately in the following texts (ICPTN) (171). Macroscopically “flat” (nontumoral) dysplasia is typically difficult, if not impossible, to distinguish from acute or chronic cholecystitis changes. It may be associated with mucosal congestion, plaque formation, feathery change, or ulcerated appearance, depending on its growth pattern.
Microscopically, most cases of dysplasia have the nonproliferative flat/undulating pattern (Fig. 38.30) (223). This is followed by those associated with glandular arrangement (Fig. 38.31). These two patterns need to be recognized by their cytologic characteristics because they do not display any significant architectural complexity. Having said that, true dysplasia of the gallbladder is typically associated with nucleomegaly, hyperchromasia, high nuclear-to-cytoplasmic ratio, cytoplasmic chromophilia, and prominent nucleoli, which altogether renders it recognizable at low-power examination. In fact, we find it to be a good attestation to the dysplastic nature of an atypical focus that it is recognizable at low power. Dysplasia with more complex patterns such as micropapillary/tufting (Fig. 38.32) and tall papillary types are easier to recognize in this regard. Of note, reparative atypia in the gallbladder seldom leads to such architectural complexity. On the opposite end of the spectrum, some gallbladder dysplasia has a denuding pattern (Fig. 38.36), with only a few cells clinging to the stroma, akin to those “denuding CIS” seen in the urothelium. This denuding/clinging pattern occurs in two settings. The first is in areas of hemorrhage and ulceration. Individual hyperchromatic clinging cells in this setting ought to be examined carefully. The second is in hyalinizing cholecystitis. In the context of fully established hyalinizing cholecystitis (where the entire wall has transformed into paucicellular fibrosis, with no muscularis or other layers discernible), any remaining epithelium ought to be regarded suspect for neoplasm.
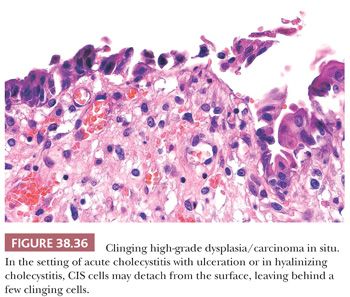
Various cellular types have been recognized in the dysplasia of the gallbladder (223,224). Most high-grade dysplasia cases exhibit enlarged cuboidal cells with a fair amount of cytoplasm, a relatively monotonous appearance, and prominent nucleoli. This is referred to as biliary cuboidal type (Fig. 38.37). The cytoplasm is often chromophilic (typically acidophilic) but in about 10%, it is exclusively clear; and in fewer cases, there is a halo around the nucleus creating a pattern similar to “chromophobe” pattern seen in renal cancers. Yet a smaller percentage of biliary pattern cases show striking oncocytoid cytology. Biliary cuboidal cell type is often relatively easy to recognize in the setting of intact mucosa, but a similar cytologic change can be seen in some cases with acute injury and ulceration. Denuding CIS in this setting can be difficult to distinguish from regenerative atypia (Fig. 38.11). Marked nuclear enlargement, severe hyperchromasia, and prominent acidophilic nucleoli are helpful (Figs. 38.30 to 38.32 and 38.37 to 38.39).
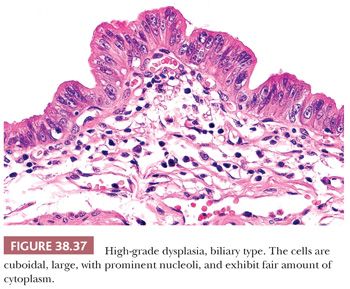
Some dysplasia of the gallbladder has more columnar cytology (223,225). These are characterized by pseudostratification of elongated nuclei with relatively minimal cytoplasm, creating an overall basophilia recognizable in low-power examination (Figs. 38.38 to 38.41). The nucleoli are often conspicuous or inapparent. Some have more thin, slender, elongated nuclei and cytoplasmic characteristics reminiscent of the “pencillate” cells of gallbladder (Figs. 38.7 and 38.38), whereas others show more cigar-shaped nuclei and cytoplasmic features that create a pattern highly similar to that seen in adenomas of the GI tract, referred to as intestinal type (Fig. 38.39). Goblet-type cells are seen in dysplasia not uncommonly, both in this type and others, and appear to be a sign of maturation. The presence of goblet cells should alert pathologists to look for dysplasia because the association seems to be fairly strong. Other metaplastic cell types such as neuroendocrine and even pancreatic acinar may also be seen occasionally in dysplasias.
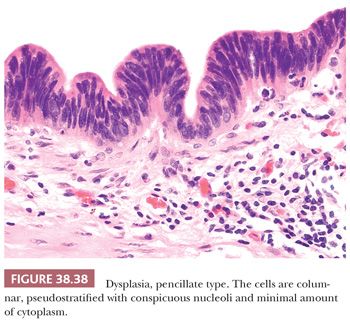
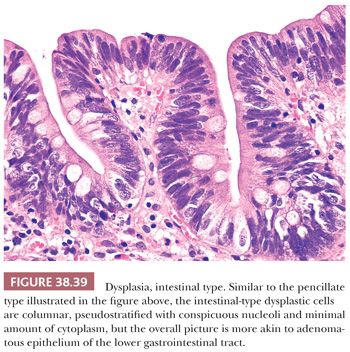
Perhaps the most challenging among cell types of dysplasia is the one characterized by tall columnar mucinous cells that have abundant apical cytoplasm that contain gastric or endocervical-like mucin (Fig. 38.40). In this pattern, the nuclei are often well polarized at the base of the cells and do not show overt pleomorphism and atypia typically associated with conventional dysplasia. The overall pattern becomes very bland-appearing and difficult to recognize as dysplasia. On the other hand, considering that normal and reactive gallbladder epithelium does not show any significant intracellular mucin, any areas with voluminous mucinous cytoplasm should be examined carefully with the suspicion of this subtle but often aggressive form of dysplasia (223,225).
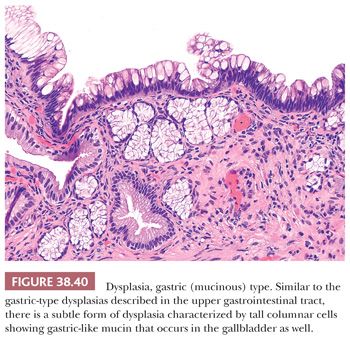
Signet ring cell and squamous varieties of dysplasia are exceedingly rare. In fact, cases with these patterns in the surface epithelium typically prove to be cancerization (colonization) of the surface mucosa by the underlying invasive carcinoma. It ought to be kept in mind also that there is a peculiar form of mucosal degeneration that can form atypical signet ring–type cells (226).
In the approach to the diagnosis and differential diagnosis of dysplasia, in addition to the features described earlier, a few additional points may be helpful. If focal epithelial atypia is encountered in the context of well-preserved unremarkable epithelium, this commonly proves to be regenerative atypia. True high-grade dysplasia appears to spread quickly to involve most of the preserved epithelium (wildfire phenomenon) by the time of cholecystectomy. The main exception to this is hyalinizing cholecystitis where any epithelium in the context of dense paucicellular fibrosis (without any normal tissue remaining) ought to be regarded suspect for carcinoma. In cases with focal epithelial atypia seen in intact mucosa, we recommend two to four additional blocks with multiple strips in each to exclude a more sinister process. These reactive atypia typically display round to ovoid overlapping cells with prominent intercellular clefts, molding of nuclei, attenuated appearance (Fig. 38.41), and overall basophilia. This often occurs at the deeper aspects of the epithelium with maturation towards the surface. Nuclei have relatively fine and diffuse chromatin, and the nucleoli are typically small or conspicuous. Mitotic activity is often brisk in these regenerative foci. In contrast, the reactive atypia seen in acute cholecystitis is challenging in different ways. In evaluation of such cases, it is important to keep a high threshold before rendering the diagnosis of dysplasia but a very low threshold in taking additional sections to examine.
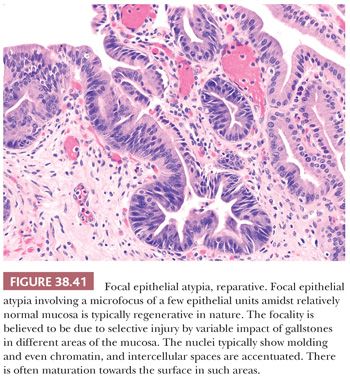
If a convincing focus of high-grade dysplasia is detected, it is crucial to perform thorough sampling, with a minimum of 12 cassettes and preferably the entire gallbladder. This is because even a superficial invasion into perimuscular tissue (pT2), which is very difficult, if not impossible, to detect by gross examination of the specimen, confers much more aggressive behavior. If a pT2 has been ruled out by thorough sampling, then early gallbladder cancers appear to have a very good prognosis with 10-year survival of 90% unless there is Rokitansky-Aschoff involvement (16). In contrast, the studies based on databases with random sampling disclose a different, much more aggressive picture for early cancers, attributed to the undersampling phenomenon with missed advanced cancers.
Based on the early gallbladder cancer data (16), it is believed that the prognosis of in situ carcinoma is very good, with most cases cured of their disease by cholecystectomy. This is also supported in our analysis of Surveillance Epidemiology End Results (SEER) database of United States government, as well as our own patients (204,205). These data indicate a 10-year age-adjusted survival of 80% to 90% for CIS cases. At the same time, however, all of this data also indicates that a small proportion of the patients experience recurrences and metastasis. In some cases, this progression is encountered soon after the diagnosis, attributed to missed foci of invasive carcinoma or an unusual rapid progression phenomenon. In many cases, however, the progression occurs several years after the diagnosis, in some, after a decade, indicating that there is a field effect phenomenon, rendering the remainder of the biliary tract at risk for carcinoma. It appears that cases with multifocal or extensive CIS, those with CIS involving the Rokitansky-Aschoff sinuses and margin positivity, may confer additional risk for such recurrence, but more studies are needed to confirm this impression.
II. TUMORAL INTRAEPITHELIAL NEOPLASMS
Intracholecystic Papillary Tubular Neoplasms (Adenomas and Intracystic Papillary Neoplasms)
Previously recognized under eight different names (pyloric gland adenoma, intestinal adenoma, biliary adenoma, tubulopapillary adenoma, papillary adenoma, tubular adenoma, papillary neoplasm/adenocarcinoma, papillomatosis) by Albores-Saavedra and colleagues (7,67,151,312), mass-forming preinvasive neoplasms of the gallbladder were later regrouped under the headings of “Adenoma” and “Intracystic Papillary Neoplasms” in the WHO 2010 (187) and more recently, proposed to be unified under the category of Intracholecystic Papillary Tubular Neoplasms (ICPTN) (171). As such, in many respects, ICPTN can be regarded as the gallbladder counterpart of intraductal neoplasms of the pancreas (intraductal papillary mucinous neoplasms and intraductal tubulopapillary neoplasms) or bile ducts (intraductal papillary neoplasms and intraductal tubulopapillary neoplasms) discussed in later sections.
In the literature, the term adenoma had been used mostly synonymously with pyloric gland adenoma, and most of the cases documented were composed of subcentimeter collections of pyloric-type glands, with the average size in the largest series ranging from 0.7 to 0.9 cm (including lesions as small as 0.2 cm) (168–170). These would now be regarded as polypoid metaplasia. These data, not surprisingly, drew an entirely innocuous picture for these so-called adenomas, although even in those studies, cases that are larger than 1 cm (Fig. 38.42) had been found to have different biology, not uncommonly showing cancerous transformation. When these more clinically relevant examples of this category are evaluated separately—and for definition of clinical relevance, the arbitrary size of 1 cm was selected, extrapolating from the definition used for pancreatic/biliary intraductal neoplasms, and it is also widely used as the cholecystectomy indication—then the following clinicopathologic characteristics become apparent:
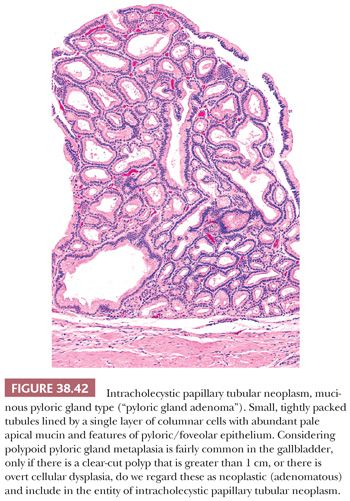
These more-than-1-cm examples occur in patients in their 60s (171). They present with nonspecific symptoms, and some are discovered incidentally. Gallstones are relatively uncommon. They occur more commonly in the fundus or body. Radiologically, about 50% are designated “cancer” and sometimes mistaken as stones. Rare cases have been reported in association with Peutz-Jeghers or Gardner syndrome (181,227–230), anomalous union of pancreatobiliary ducts, and Brunner gland hamartoma. There appears to be geographic differences in the incidence of these lesions (231).
Macroscopically, some ICPTNs especially the subset composed of complex tubular pattern (see the following texts) is typically pedunculated with a very thin, fragile stalk, so much so that the polyp is often detached from the surface, and present as “debris” in the lumen (178) (Fig. 38.43). It is also this subset that is almost always devoid of either acute or chronic changes in the background gallbladder, and thus, careful sampling of the luminal debris becomes the only means to the diagnosis of this variant. In contrast, more papillary examples are typically sessile and show more vermiform pattern, corresponding to the villous/papillary architecture (Fig. 38.44).
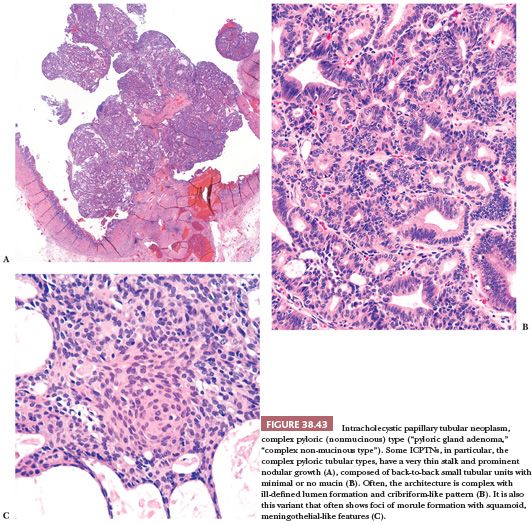
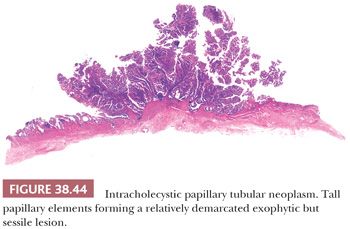
Microscopically, these tumors are characterized by compact, exophytic lesions composed of back-to-back, tubular, papillary, or tubulopapillary units in variable mixture (171) (Figs. 38.43 to 38.47). Four cell types can occur and are seen in a mixture (171). Most show cuboidal-to-columnar cells with inconspicuous nucleoli, creating a pattern akin to the pancreatobiliary type of pancreatic intraductal papillary mucinous neoplasms and thus termed biliary (Fig. 38.48). These often show MUC1 positivity. They can be associated with significant amount of inflammation, lymphoplasmacytic and/or neutrophilic, both in the cores of the papillae as well as within the epithelium in some cases (Fig. 38.48). Some cases show tall columnar cells with abundant pale mucinous cytoplasm, reminiscent of gastric foveolar or endocervical epithelium, and express MUC5AC. These often show transition to the biliary phenotype if carefully examined (Fig. 38.46). Although they appear relatively bland cytologically, they are often accompanied by more conventional high-grade dysplasia and invasive carcinoma. Some ICPTNs have more columnar cytology with elongated nuclei, but true intestinal lineage of the type described in intestinal-type intraductal papillary mucinous neoplasms, and resembling adenomas of the GI tract, are relatively rare (<10% of the cases) (Figs. 38.45 and 38.47), especially if one seeks to substantiate them by the expression of MUC2 and CDX2. Other rare cases with oncocytic cytology or urothelial-like pattern also occur.
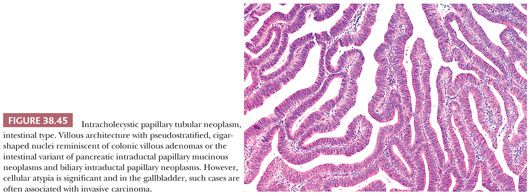
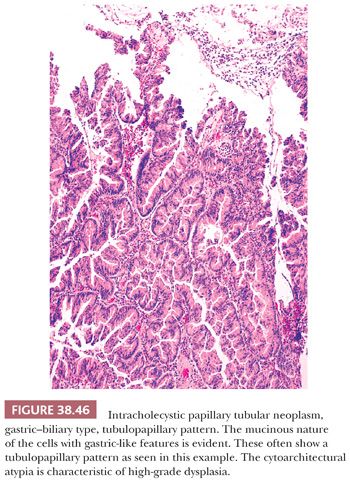
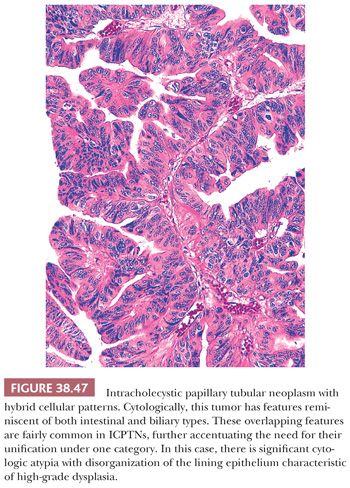
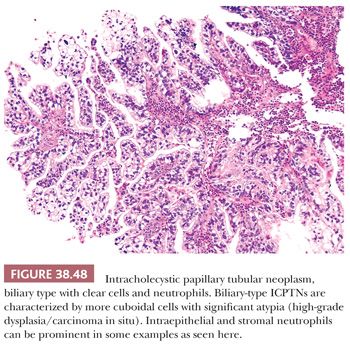
A highly distinctive subset is composed of small round tubular units in a complex architecture (178), lined by cuboidal cells with minimal or no intracytoplasmic mucin. We designate this group as complex tubular nonmucinous type. These typically show the complexity and the atypia (Fig. 38.43) that warrants the designation of carcinoma and are often diagnosed as such by experts (232), although they appear to be the mucin-poor version of the so-called pyloric gland adenomas, as evidenced both by diffuse MUC6 staining, their architectural pattern, squamoid morule formation (Fig. 38.43C), and occasional cases showing a mixture with more conventional mucinous pyloric gland subset. The glandular units in the mucinous examples are indistinguishable from normal pyloric glands (Fig. 38.42), Brunner glands, or peribiliary mucous glands when taken in isolation; and for this reason, for the definition of their neoplastic nature and their distinction from polypoid metaplasias, the size criteria of 1 cm has been employed (171). In fact, the literature on the so-called pyloric gland adenomas drawing an entirely innocuous picture is mostly due to the fact that most of the studies were based on polypoid metaplasias as small as 2 to 3 mm (168–170).
Both complex tubular as well as the mucinous variants of pyloric-type cases typically have a thin stalk (Fig. 38.43), and nodular polypoid pattern, and occur in uninjured gallbladders, with the exception of cholesterolosis. In fact, cholesterolosis is noted within the lesion in many of the complex tubular cases, which, combined with the overall architecture, gives the impression that they may be arising in association with cholesterol polyps. Squamoid morules (Fig. 38.43C) are seen in more than two-thirds and may contain biotin-rich optically clear nuclei (171,233). Complex tubular examples can have cystically dilated glands with acidophilic secretions. Focally, hyalinized amyloid-like stroma may be encountered. The surface epithelium covering the individual nodules is often unremarkable, further accentuating the similarity to the architecture of cholesterol polyps.
Although the complex tubular ones exhibit marked architectural and some cytologic atypia warranting the diagnosis of carcinoma, they are seldom, if at all, associated with invasive carcinoma, further supporting that they are in fact a complex version of the so-called pyloric gland adenoma. As such, they can also be regarded as gallbladder kindreds of pancreatic/biliary intraductal tubular (tubulopapillary) neoplasms (187,234,235). In contrast, papillary/villous examples are more prone to have invasion and exhibit aggressive behavior.
These tumors, in essence, represent the adenoma–carcinoma sequence pathway of carcinogenesis. An estimated 6% of invasive carcinomas of this organ arise in association with these ICPTNs (171). A spectrum of dysplasia can be seen both within the category and often in a given case. The grading of dysplasia is similar to the grading employed for flat (nontumoral) lesions discussed earlier.
WHO 2010 separates these tumors into two distinct categories of either adenoma or intracystic papillary neoplasm; however, it is becoming clear that overlaps are too common, and in fact, a recent study disclosed that there is virtually no agreement among experts in separating these tumors into one of these two categories (232). Thus, the unifying category of ICPTNs was created (171), extrapolating from the pancreatic and biliary counterparts (187). In reporting these cases, more important than the category designation is to rule out invasive carcinoma by extensive if not thorough sampling. If there is invasion, its characteristics, including the size, ought to be documented, separately from the noninvasive component, as for any other invasive carcinoma. For the noninvasive component, the size ought to be recorded along with the extent of high-grade dysplasia, the cell type (if can be determined), and configuration (tubular, papillary, or tubulopapillary), if possible. Studies have shown that those with extensive high-grade dysplasia and papillary configuration and biliary or foveolar phenotypes are more prone to have invasive carcinoma and aggressive behavior (171). Invasive carcinomas, seen in 65% of the cases, are most commonly conventional tubular (pancreatobiliary) type, although occasionally, it may be neuroendocrine, squamous, sarcomatoid, or undifferentiated as well (171).
Due to the architectural complexity of the lesions, it is often difficult to determine the presence and degree of invasiveness in these cases. Many examples have the complexity that can be regarded as intramucosal adenocarcinoma. Unless there is convincing invasion into the stroma, we classify the case as high-grade dysplasia/CIS. However, this diagnosis can only be rendered after examination of multiple levels and thorough (if not entire) sampling of not only the polypoid/papillary lesion itself but also the uninvolved gallbladder because insidious invasive carcinomas can be found in the remainder of the gallbladder, away from the ICPTN lesion, unrecognized grossly (171). For cases that remain indefinite for invasion despite thorough workup, the principles discussed earlier for “early gallbladder cancers” should be employed (16).
In terms of prognosis, noninvasive examples seem to have a much better outcome than those with invasion (reported 3-year survival of 90% vs. 60%) (171). However, there are cases that are classified as noninvasive and still follow an aggressive clinical course. Early progression seen in these seemingly noninvasive cases are attributed to missed foci of invasion (thus the importance of thorough sampling), but the late recurrences are believed to be due to a field effect phenomenon affecting the biliary tract, and for this reason, long-term follow-up is recommended. Those with invasive carcinoma have significantly worse prognosis than the noninvasive group but still much better than the non–ICPTN-associated ordinary gallbladder adenocarcinomas (171). This observed survival benefit over the conventional (flat dysplasia–associated) carcinomas, which is also true for the pancreatic and biliary counterparts of these tumors (187,234,236–240), may be attributable to two factors:
1. Their mechanism of pathogenesis is different, making them a distinct, more indolent pathway of carcinogenesis (adenoma–carcinoma sequence) (241–243).
2. They are detected and treated in early stages. However, in some studies, the survival advantage persisted even when stage-matched (171), suggesting a biologic rather an early detection phenomenon.
Not surprisingly, molecular alterations identified in these tumoral intraepithelial neoplasms (adenoma–carcinoma sequence) are fairly different from those observed in the conventional dysplasia–carcinoma sequence. Mutation of the β–catenin gene is detected in 60% of pyloric gland subset (242), although it is uncommon in other subtypes. Mutation of K-ras oncogene (241) also seems to be more common and p53 mutation less common than in ordinary dysplasia–carcinoma sequence. GNAS mutation in codon 201, which is seen in more than 60% of pancreatic intraductal neoplasms, is uncommon in these tumors (244,245). Rarity of intestinal phenotype may account for this difference because this mutation seems to be much more common in tumors of intestinal lineage (245).
III. MUCINOUS CYSTIC NEOPLASIA
Mucinous cystic neoplasms of the type that is well characterized in the pancreas (246,247), liver (248), and extrahepatic biliary tree (249) are exceedingly uncommon in the gallbladder proper but may present like a gallbladder lesion due to secondary growth. These tumors will be discussed in detail later in “The Extrahepatic Bile Ducts” section.
INVASIVE CARCINOMAS
Adenocarcinoma
Conventional tubular-type adenocarcinoma of the gallbladder, essentially regarded as a pancreatobiliary-type adenocarcinoma due to its morphologic and behavioral kinship with pancreatic ductal carcinoma and cholangiocarcinoma, is the most common malignant neoplasm of this organ and therefore, most attributes of “gallbladder cancer” are related to this type of carcinoma.
Epidemiology, Risk Factors, and Clinical Findings. The gallbladder is the most common site for malignancy within the biliary tract and sixth among GI tract–related organs (250,251). Approximately 6000 Americans are diagnosed with this cancer every year (252). There is a striking female predominance (16,250,251,253), approximately 3:1 ratio, and most patients are older than 50 years, with the mean age older than 64 years (322–324). It is significantly more common among Native Americans and Latin Americans than whites or African Americans. There is a definite epidemiologic parallel between gallbladder carcinoma and cholelithiasis, and the high incidence of the former in these populations may be partly attributable to their propensity to develop cholelithiasis, especially cholesterol stones. In the literature, porcelain gallbladder, which had been defined as extensive calcification in the gallbladder wall was reported to have strong association with carcinoma, but recent studies have shown that it is actually a distinctive type of hyalinizing cholecystitis with minimal or no calcifications (incomplete porcelain) that has high risk for carcinoma (23,98,103). Some of the GI disorders that have a predisposition for gut carcinomas such as ulcerative colitis (105,254) and polyposis syndromes (228,255) also have an association with gallbladder adenocarcinoma. Gallbladder is also being increasingly appreciated as an organ afflicted in Lynch syndrome (256,257). The incidence of gallbladder carcinoma is also high in patients with primary sclerosing cholangitis (258). Abnormal choledochopancreatic junction (conjugation of CBD with pancreatic duct outside the wall of the duodenum), which is often associated with choledochal cysts, is another factor associated with gallbladder carcinoma (221,222,259,260), presumably due to the reflux of pancreatic secretions unchecked by the sphincter of Oddi. Of note, carcinomas arising secondary to malunion appear to occur in younger patients (mean, age 55 years) with striking female predominance and often in the absence of gallstones. Similarly, reflux may also be a factor in the development of carcinomas in choledochal cysts (222,261,262) (see the following section). Interestingly, the carcinomas associated with this anomaly have a high incidence of ras mutation as opposed to others (263–265).
Some infectious agents (266), especially S. typhi, have been implicated in gallbladder carcinoma, presumably due to its long-term inhabitance in bile and the potential for altering the bile salts into carcinogenic agents (267–270). There are recent studies investigating the role of Helicobacter species (94,271,272), especially Helicobacter bilis (273,274).
The signs and symptoms of gallbladder cancer are nonspecific, mimicking chronic cholecystitis (pain, anorexia, elevated alkaline phosphatase), and manifest only after the tumor has inflicted substantial damage (275). Upper abdominal pain and increased serum alkaline phosphatase levels are the most common findings at presentation. Rarely, paraneoplastic syndromes may be encountered. As is typical of pancreatobiliary-type carcinomas, CA19-9 serum level is often very high and is a useful marker to track disease progression. Carcinoembryonic antigen, (276,277) and, rarely, alpha-fetoprotein (277,278) may also be elevated.
A substantial percentage of gallbladder adenocarcinoma is detected in cholecystectomies performed with the preoperative diagnosis of acute or chronic cholecystitis, also referred as inapparent (253,279). For this reason, routine sampling of cystic duct margin is important, especially considering that these clinically unsuspected carcinomas tend to be more commonly located towards the neck of the organ (280). These clinically unsuspected carcinomas removed by nononcologic operations such as laparoscopic cholecystectomy may also be a source of seeding along the endoscopic tract or spillage and intra-abdominal dissemination.
Macroscopic Features. Gallbladder adenocarcinoma typically produces a scirrhous, gray-white, firm mass (Fig. 38.49) due to the prominence of desmoplasia. The intraluminal component may be polypoid (281). When diffuse, it may be difficult to distinguish from cholecystitis, hence the importance of microscopic examination of every resected gallbladder. Diffuse infiltrative types may create a picture reminiscent of linitis plastica and may spread linearly along the extrahepatic bile ducts as well. Most carcinomas arise in the fundus (60%) followed by the body (30%) (275); those in the neck (10%) often grow into the cystic duct, and the precise origin may be difficult to determine (280). Calculi are present in more than 80% of the cases. Extension into the liver, which is common, may result in encasement and even obliteration of the gallbladder (282). The component that grows towards the liver may appear deceptively well demarcated.
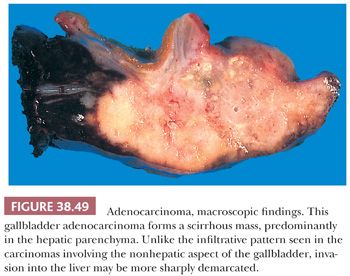
Microscopic Features. Conventional gallbladder carcinoma is mostly of tubular type and displays the typical features of pancreatobiliary-type adenocarcinoma, characterized by well-formed glands with wide lumina (Figs. 38.50 and 38.51) that are lined by a layer of cuboidal cells and often embedded in a desmoplastic stroma (275). Often, the nuclear grade is unexpectedly high for the degree of glandular differentiation, although some cases have deceptively bland cytologic features. The cytoplasm may be acidophilic and granular in some cases or pale or clear in others. Mucin, in particular sialomucin (283), nonsulfated, or neutral types, is demonstrable by histochemical or immunohistochemical stains in almost all cases and may be abundant, both intracellularly and extracellularly. Goblet cells, Paneth cells, and neuroendocrine cells may be identifiable (7,284,285).
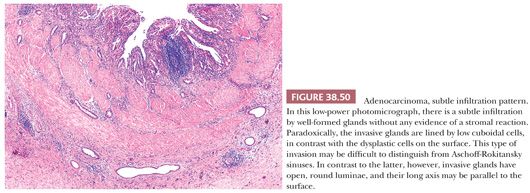
Perineurial (Fig. 38.51) and vascular invasion are common. Even in perineurial and vascular spaces, the tumor cells may be very bland and may form well-defined glandular structures.
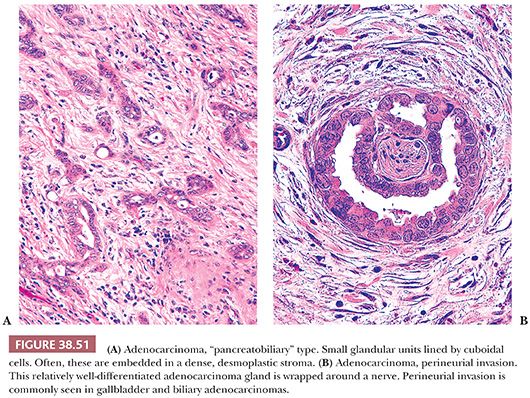
Immunohistochemical Features and Ancillary Studies. The diagnosis of gallbladder adenocarcinoma is morphologic. Immunohistochemical stains do not reliably distinguish invasive carcinomas from noninvasive neoplasms or benign reactive conditions. Also, there are no established specific markers to distinguish gallbladder adenocarcinomas from other foregut-origin carcinomas, in particular, pancreatic and biliary tract cancers. Overlaps are also very common with upper GI, müllerian, and pulmonary cancers.
Gallbladder adenocarcinomas usually express carcinoembryonic antigen (CEA), CA19-9 (286), CEA (often intracytoplasmic), MUC1, epidermal growth factor receptor, p53, B72.3, MUC6, and cytokeratins (3,7,288). Among cytokeratins, CK7 is commonly expressed, whereas CK20 is less common. Estrogen receptors are detectable in a small percentage as well (289). Scattered neuroendocrine cells, positive for chromogranin and synaptophysin stains, may also be found. Immunohistochemical expression of p53 is detected in two-thirds of the cases and may aid in the differential diagnosis with benign mimics but ought to be used cautiously due to significant overlap.
Molecular and genetic studies have revealed that mutations and loss of heterozygosity in the p53 gene is very common in gallbladder carcinomas (195). Loss of heterozygosity of 8p, 9p, and 18q (195,290,291) and amplification of c-erb-B2 are detected in close to half of the tumors (292). Mitogen-activated protein kinase pathway alterations seem to be the most common (293–295). Recently, mTOR pathway alterations have been found to be associated with adverse prognosis (296). Although gallbladder carcinoma is regarded as a pancreatobiliary-type adenocarcinoma by morphology, the mutation in the K-ras oncogene (297), which is present in the vast majority of pancreatic ductal adenocarcinomas, is very rare in gallbladder carcinoma (298). Similarly, loss of DPC4 is also much less common (299,300). Microsatellite instability is reported in 10% of the cases (301). Most gallbladder carcinomas are aneuploid (302,303). In some studies, COX-2 overexpression was found to have correlation with prognosis (304,305). There are also various epigenetic alterations, including methylation of tumor suppressor genes. Some of these are reported to show geographic differences in occurrence (306).
Morphologic Patterns of Adenocarcinoma. Conventional gallbladder adenocarcinoma may exhibit some morphologic variations (7,187,307). These do not appear to be biologically or clinically distinct from ordinary gallbladder carcinoma and most are associated with the conventional pattern in other parts of the tumor. In some cases, the tubular units have morphologic characteristics of foamy gland adenocarcinoma (Fig. 38.52) described in the pancreas (308). This variant is also referred to as the clear cell pattern (7,309); however, the cytoplasm is not clear but rather foamy or microvesicular with evenly sized, minute vesicles. Often, there is a distinct chromophilic band in the apical cytoplasm. The nuclei may be indented by the vesicles of the cytoplasm and may acquire a pyknotic, raisinoid appearance. These features are critical in distinguishing this variant of carcinoma from benign pyloric-type glands. In some examples of this variant, tubule formation is not as evident, and the invasive carcinoma cells form solid clusters in the stroma, which may be mistaken for foamy macrophages. A variation on the theme is those cases that have gastric foveolar-like appearance showing pale, acidophilic, finely granular, or homogenous cytoplasm without cytoplasmic vesicularity or brush border–like cuticle. These appear extremely bland cytologically, and the diagnosis may have to be based on the pattern of spread. Similarly, some cases are characterized by exceedingly well-formed small glandular units lined by bland, low cuboidal cells, similar to the tubular carcinomas of the breast. In this variant, the presence of nuclear grooves, paucity of cytoplasm, and pattern of spread especially the formation of open round lumina and an axis parallel to the surface are the features that would help distinguish them from Rokitansky-Aschoff sinuses (310). Some gallbladder carcinoma cases exhibit clear cytoplasm and a more alveolar growth pattern (see the following texts and Fig. 38.53). In another morphologic variant of gallbladder carcinoma, the nuclei are attenuated; and when combined with the branching architecture of the glandular elements, a pseudoangiomatous pattern is exhibited (307). A variation on this theme is the acantholytic change in sheetlike areas forming a pattern resembling the Masson phenomenon (intravascular papillae) in angiosarcomas. In some cases, gallbladder adenocarcinoma may exhibit more columnar cells with stratification (intestinal features). This pattern is rarely the predominant pattern, and the intestinal appearance is seldom as well developed as in true intestinal adenocarcinomas. Therefore, if a carcinoma in this region exhibits very characteristic intestinal morphology, a secondary tumor ought to be considered in the differential (see the following section). Other examples of gallbladder carcinoma have a cordlike infiltration pattern, mimicking invasive lobular carcinomas of the breast. A subset has a more nested appearance mimicking the so-called “alveolar” variant of this tumor. Gallbladder adenocarcinoma may also form cribriform units (307), and in some, the invasive elements are relatively large and exhibit complex papillary configurations, similar to the so-called “invasive papillary carcinomas” that occur in the ampulla (see the following section). Rarely, a gallbladder adenocarcinoma may contain choriocarcinoma-like areas (7).
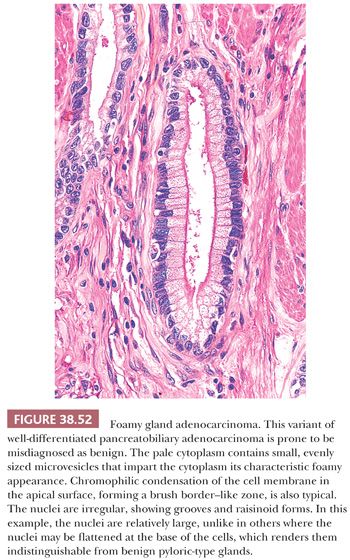
Differential Diagnosis. Gallbladder adenocarcinomas may be deceptively bland-appearing and may be difficult to distinguish from Aschoff-Rokitansky sinuses or Luschka ducts (see histology of gallbladder) (11,16). Nuclear enlargement, nuclear contour irregularities, hyperchromatism, loss of polarity, mitotic figures, apoptotic cells, intraluminal necrosis, intraluminal neutrophils, open luminae, and microvesicular cytoplasm with a distinct thin apical chromophilic band are findings in favor of carcinoma. Fibrosis around the epithelium may not be helpful because it may be present in both (Fig. 38.54). Aschoff-Rokitansky sinuses often appear to be continuous with the surface epithelium and have a flask shape or axis perpendicular to the surface. Benign epithelium usually has undulating contours, compressed lumina, and columnar appearance, whereas most carcinomas have open round lumen formation (Fig. 38.50), contour irregularities, and cuboidal epithelium with a distinct cytoplasmic color. Luschka ducts can become proliferative and appear pseudoinfiltrative in cases with gallbladder inflammation (Fig. 38.55) (11). These proliferative examples of Luschka are similar to biliary ductular proliferations seen in the liver. They may show mitotic activity. They are usually small uniform units confined to the hepatic surface of the gallbladder and do not exhibit irregular size or shape, the round and open lumina or nuclear grooves characteristic of well-differentiated adenocarcinomas. A bandlike pattern confined to the subserosa and lack of vertical growth with connection to the surface are characteristic of Luschka ducts, but some carcinomas can also exhibit lateral spread in the subserosa forming a band, and the connectivity to the mucosa may have to be documented in additional sections (20,311). None of the molecular and immunohistochemical markers, including p53 and Ki-67, has a conclusive role in this distinction because overlaps are too common.
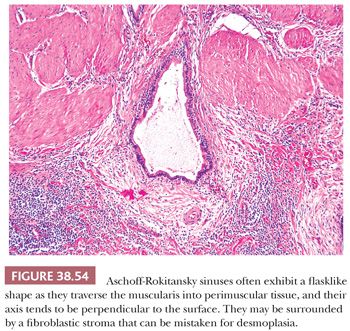
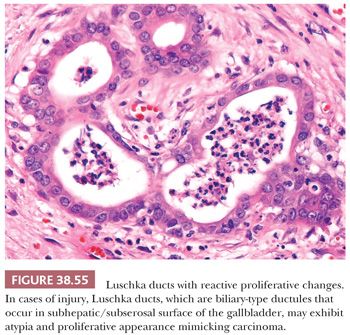
Other Types of Invasive Carcinoma
The following carcinomas of the gallbladder are regarded somewhat separately from ordinary adenocarcinomas because they show distinctive morphologic characteristics beyond the realm of the conventional pancreatobiliary-type features described earlier.
Having said this, many of these are commonly associated with ordinary adenocarcinoma in the background; and for this reason, these designatives should be used only if they are the predominant pattern.
So-Called Papillary Adenocarcinomas. As discussed in the ICPTN section earlier, papillary adenocarcinomas (312) (Fig. 38.44) of preinvasive nature are better regarded within the conceptual category of preinvasive neoplasia (171). However, it should be acknowledged that these are commonly associated with invasive carcinoma and have an aggressive clinical course. They should not be confused with the invasive papillary carcinomas, which are a type of invasive carcinoma that forms papillary elements within the invasive units (312).
Intestinal Type. Intestinal-type adenocarcinoma with all the features of conventional colonic adenocarcinomas including central necrosis, goblet cell–like mucin, and cellular basophilia is very uncommon in this anatomic region. In fact, if one is encountered, the possibility of metastasis from colon ought to be excluded first. However, ordinary gallbladder adenocarcinomas may on occasion exhibit foci of columnar cells and pseudostratification and, thus, resemble intestinal carcinomas. These foci may also show immunophenotypic evidence of intestinal differentiation such as CDX2 and MUC2 expression, although these markers may be expressed in otherwise usual examples of gallbladder adenocarcinomas as well. In general, we advocate not classifying these tumors as intestinal adenocarcinomas unless they show all the characteristics of colonic adenocarcinomas throughout. Separately, a rare distinctive type of gallbladder carcinoma showing more complex growth pattern with back-to-back glandular arrangement has also been classified as “intestinal” in the literature (7,8,313). Another very rare, and rather peculiar, well-differentiated form of adenocarcinoma that occurs in this region shows resemblance to intestinal mucosa and has also been categorized as “intestinal type.”
Squamous Cell and Adenosquamous Carcinomas. Squamous differentiation can be encountered in 7% of gallbladder adenocarcinomas (314). Those with squamous differentiation in less than 25% of the tumor are reported as focal squamous change; those with 25% to 99% qualify as adenosquamous carcinoma. Pure squamous cell carcinoma without any glandular component is highly uncommon, constituting about 1% of carcinomas of this organ. Squamous and adenosquamous carcinomas often appear necrotic grossly. They also tend to have significant amount of inflammation, which may be the reason why most are mistaken as cholecystitis at the time of diagnosis. Giant cell formation is also common (314). They may be associated with squamous metaplasia as well as squamous and glandular dysplasia. Squamous differentiation can be represented in various patterns, ranging from prominent keratinization to poorly differentiated with a pavement pattern or intercellular junctions as the only evidence. Spindle cell (sarcomatoid) and clear cell variants also do occur (7,315–317), admixed with more conventional squamous areas. In our experience, squamous/adenosquamous carcinomas are highly aggressive, even more so than ordinary adenocarcinomas of this organ (314). This is in parallel with their more aggressive behavior noted in the pancreas (318). This was partly attributed to the higher stage at the time of diagnosis (319). However, in some studies, their adverse outcome persisted even in stage-matched cases (314). At the same time, some patients who survived beyond 2 years followed a more indolent course (314).
Small Cell and Other High-Grade Neuroendocrine Carcinomas. Poorly differentiated carcinomas with prominent neuroendocrine differentiation account for 4% of gallbladder carcinomas (307,320–322). They are defined by the histologic criteria applied to their counterparts in the lung, characterized by diffuse or nested growth patterns, cells with a high nuclear-to-cytoplasmic ratio, finely stippled chromatin, high mitotic activity (>10 mitoses per 10 high-power fields [hpf], by definition, and typically >30 per 10), as well as necrosis. Small cell carcinomas are defined by their morphologic features; immunohistochemical evidence of neuroendocrine differentiation is not required; chromogranin may be focal or weak and appear only as fine granules within the cytoplasm. Undifferentiated carcinomas that lack the distinctive nuclear and other morphologic characteristics should not be classified as small cell carcinoma. In distinguishing large cell neuroendocrine carcinomas from mere poorly differentiated carcinomas, the detection of one of the three markers (chromogranin and synaptophysin or CD56) may be necessary. CD56 by itself ought to be used cautiously for this purpose. Poorly differentiated neuroendocrine carcinomas are often admixed with conventional adenocarcinomas. Half of small cell carcinomas are pure, but a significant proportion of large cell type are mixed (320,322). Additionally, they are often associated with conventional dysplasia and ICPTN on the surface mucosa (322). Poorly differentiated neuroendocrine carcinomas are highly aggressive tumors with a rapidly fatal course (322), with an overall prognosis that is worse than ordinary adenocarcinomas; however, they tend to be sensitive to chemotherapy with cis-platinum–based protocols.
Poorly Cohesive Cell Carcinomas (with or without Signet Rings). Tumors with the morphologic characteristics and diffuse/infiltrative pattern typical of gastric “signet ring cell carcinomas,” now termed as poorly cohesive cell carcinoma by the WHO 2010, may be seen in the gallbladder and constitute about another 4% of cases (7,307,323). Most patients are women. Grossly, there may be a linitis plastica–type picture. These tumors are characterized by dyshesive cells, infiltrating into the stroma in forms of individual cells or cordlike pattern. True signet ring cytology is seen in virtually every example but may be very focal. Some cases are predominantly plasmacytoid, akin to their counterparts in the urinary bladder. Focal mucinous component can be seen in some cases, but the term signet ring cell (poorly cohesive cell) carcinoma should be reserved for only those tumors with the malignant cells lying individually or in clusters in the stroma; those composed of malignant cells floating within mucin lakes should be classified as mucinous carcinoma (324). Rare cases have predominantly intramucosal growth. It should also be kept in mind that diffuse infiltrative (cordlike) growth pattern is not uncommon as a focal finding in the poorly differentiated components of conventional (pancreatobiliary type) adenocarcinomas of this region. A tumor should not be classified as poorly cohesive cell or signet ring type unless the majority of the tumor shows the characteristic discohesive infiltrative pattern and relative preservation of normal structures. These are highly aggressive neoplasms (323).
Mucinous Carcinomas. It is not uncommon for gallbladder carcinomas to demonstrate profuse intracytoplasmic or intraglandular mucin production, but pure mucinous (colloid) carcinomas as seen in the breast, skin, or pancreas (325) are very uncommon in the gallbladder (324). However, mucinous carcinomas as described in the colon in which the areas with stromal mucin deposition constitute 50% to 90% of the tumor do occur (7,307,324) (Fig. 38.56). These present as large tumors, often with the clinical picture of acute cholecystitis, and have a gelatinous appearance on macroscopic examination. Most are mixed with other nonmucinous adenocarcinoma types or show abundant cells clinging to the stroma. Signet ring–type cells can be seen floating within the mucin, and some cases are associated with true poorly cohesive (signet ring) cell infiltration into the stroma (see earlier discussion). Calcifications can be prominent (326). Immunophenotypically (324), MUC2 expression is common in these tumors in contrast with ordinary gallbladder adenocarcinomas, but CDX2 is mostly negative. True to their origin, however, CK7 is positive and CK20 is negative in most. They do not seem to have any specific association with microsatellite instability. Mucinous carcinomas are typically large and advanced tumors at the time of diagnosis and appear to be even more aggressive than ordinary gallbladder adenocarcinomas (324).
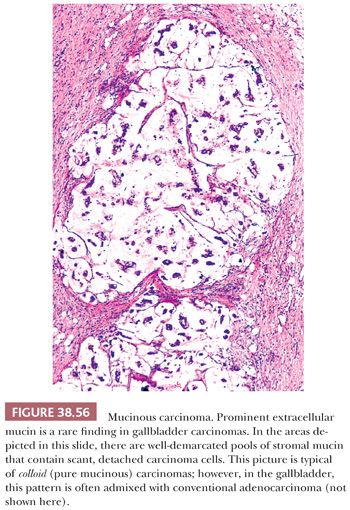
Undifferentiated Carcinoma—Epithelioid Type. Nonglandular sheetlike growth pattern can be seen in 9% of gallbladder carcinomas (307,327–329). These occur in three forms. In most cases, it shows medullary pattern with syncytial nodular growth with pushing border infiltration, often with a distinctive cytology of vesicular chromatin and prominent nucleoli and commonly associated with variable amounts (and patterns) of inflammatory infiltrate. As such, some of these cases have the features of the so-called lymphoepithelioma-like carcinoma (329). Some studies have found evidence of EBV in these tumors (330,331), whereas others have failed to do so (332). The second group is characterized by widely separated small solid rounded nests of non–gland-forming carcinoma cells. These typically do not have much associated inflammation. A rather distinctive subgroup is characterized by hepatoid morphology, showing more diffuse growth pattern and cytologic features reminiscent of hepatocellular carcinomas, along with Heppar1 positivity in some. About 10% of gallbladder carcinomas with an undifferentiated epithelioid component show microsatellite instability, and there may be an association with the hepatoid pattern (329). Cytologically, these undifferentiated epithelioid components have more monotonous rhabdoid-appearing cells. Others have an anaplastic morphology and may show multinucleated, bizarre giant cells. This may be associated with tumor cell “cannibalism” as well as inflammatory cells, neutrophils, and others, both within and around the tumor cells, reminiscent of giant cell lung carcinoma. These neoplastic giant cells should be distinguished from osteoclastic giant cell carcinomas (sarcomatoid carcinomas with osteoclasts), which occur very uncommonly in the gallbladder (see the following texts). These carcinomas are not only uniformly keratin positive but also express vimentin in almost half of the cases, not surprisingly. They seem to have a more aggressive clinical course than ordinary adenocarcinomas characterized by uniform gland formation (329).
Undifferentiated Carcinoma—Sarcomatoid Type. Spindle cell (sarcomatoid, metaplastic) carcinomas also occur in the gallbladder (307,316,317,327,333,334). As in other organs, some have a more polypoid configuration. Often, there is a distinct epithelial component (carcinosarcoma). Sarcomatoid components may be fibroblast-like or show evidence of heterologous differentiation including skeletal muscle elements, cartilage, and bone. Sarcomatoid carcinomas may lack keratin immunoexpression and the distinction from a true sarcoma may be difficult. One may need to rely upon the growth pattern and presence elsewhere of carcinoma or dysplasia. Sarcomatoid carcinomas may occasionally have rare osteoclastic giant cells, but it is exceedingly uncommon to see an osteoclast-predominant pattern (335) of the type that is well documented in the pancreas and bile ducts (Fig. 38.57). A small series of cases with similar morphology were interpreted as true (benign) osteoclastic giant cell tumor occurring in this region, mostly based on the CD163 expression of the background cells (336); however, this impression was challenged in other studies (337). In our opinion, a tumor with abundant osteoclastic giant cells in this region should be considered a sarcomatoid carcinoma unless proven otherwise.
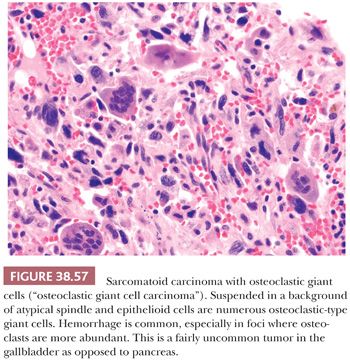
Medullary and Lymphoepithelioma-Like Carcinoma. Some poorly differentiated carcinomas of the biliary tract show a syncytial growth pattern and pushing border infiltration akin to those “medullary carcinomas” seen in the lower GI tract (329). This pattern is typically associated with nodular growth and distinctive cytology including round to ovoid large cells with vesicular chromatin and single prominent nucleoli. Whether these tumors are also associated with microsatellite instability as in their colonic counterparts needs further investigation. We have seen such an example associated with a colonic adenocarcinoma, raising this possibility. Lymphoplasmacytic infiltrates are prominent in some cases reminiscent of the lymphoepithelioma-like carcinomas seen in the upper aerodigestive tract.
Hepatoid Carcinoma. As described previously, undifferentiated epithelioid carcinomas of the gallbladder may have a hepatoid pattern (329). True primary hepatoid carcinomas of the gallbladder and extrahepatic biliary tract are exceedingly uncommon. Most hepatoid tumors in this location are either hepatocellular carcinomas arising in the liver that secondarily invade, or metastasize to this area, or are oncocytoid tumors. There are, however, rare primary carcinomas occurring in this region, associated either with surface dysplasia and/or conventional tubular adenocarcinoma, that do show a hepatoid pattern consisting of trabecular or sheetlike arrangement of polygonal-shaped cells with abundant finely granular cytoplasm and round nuclei with prominent nucleoli, with all the characteristic features of hepatocellular carcinomas. These tumors may show positivity for hepatocyte-1 antigen (Heppar-1) and for alpha-fetoprotein (278,338) as well as a canalicular pattern of staining for polyclonal CEA or CD10, supporting hepatocytic differentiation. However, unlike true hepatocellular carcinomas, they may also express CK19 and 20, which are more characteristic of biliary differentiation (338–340).
Clear Cell Carcinomas. True clear cell (hypernephroid) examples of gallbladder carcinoma, in which there are solid nests of clear cells, may also be seen (309) (Fig. 38.53). These may even have a stromal sinusoidal pattern; however, this pattern is usually associated with more desmoplastic stroma and nuclear atypia than would be expected of metastatic renal cell carcinomas. Also helpful is CK7 and CEA, which are not expressed by renal cell carcinomas. If sampled extensively, an adenocarcinoma component is often identifiable in these cases. Ordinary adenocarcinomas of the gallbladder with foamy gland (308) (Fig. 38.52), vacuolated (341), foveolar, and endocervical-like cytology sometimes erroneously receive the designation of “clear cell.”
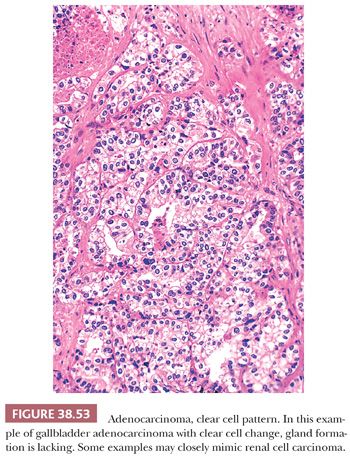
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


