1 What are fungi?
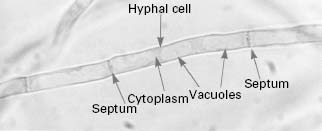
Yeast, such as brewers’ yeast, and moulds, such as Penicillium chrysogenum which produces the antibiotic penicillin, are classified as fungi. Yeast cells tend to grow as single cells which reproduce asexually in a process known as budding, although a minority of species (e.g. Schizosaccharomyces pombe) reproduce by fission. Many yeast species are capable of sexual reproduction and the formation of spores. In contrast, moulds grow as masses of overlapping and interlinking hyphal filaments and reproduce by producing masses of spores in a variety of structures. This division between yeast and moulds based on growth morphology is not clear cut since some yeast can produce hyphae under specific conditions (e.g.Candida albicans) while many normally filamentous fungi possess a yeast-like phase at some point in their life cycle. Fungi are eukaryotic organisms, i.e. their cells possess a nuclear membrane, consequently there are many similarities between the biochemistry of fungal cells and vertebrate (human) cells. Fungi are widely distributed in nature, occurring as part of the normal flora on the body of warm-blooded animals, as decomposers of organic matter and as animal and plant pathogens. Medically, fungi are an extremely important group of microbes, being responsible for a number of potentially fatal diseases in humans (Table 4.1), but a significant number of fungi are of great benefit to humanity in terms of the production of alcoholic beverages, bread, enzymes, antibiotics and recombinant proteins (Table 4.2). Fungi have also been utilized for a range of molecular biological applications.
Table 4.1 Examples of fungal diseases and selected causative agents.
| Type of mycosis | Disease | Speciesname |
| Superficial | Pityriasis versicolor White piedra | Malassezia furfur Trichosporon beigelii |
| Cutaneous | Tinea pedis (athlete’s foot) Onychomycosis (nail infection) Tinea capitis (scalp ringworm) | Trichophyton rubrum Trichophyton rubrum Trichophyton tonsurans |
| Subcutaneous | Chromoblastomycosis Mycetoma | Fonsecaea pedrosoi Acremonium spp. |
| Systemic | Blastomycosis Histoplasmosis Coccidioidomycosis Paracoccidioidomycosis | Blastomyces dermatitidis Histoplasma capsulatum Coccidioides immitis Paracoccidioides brasiliensis |
| Opportunistic | Candidosis (superficial/systemic) | Candida albicans Candida glabrata Candida parapsilosis |
| Aspergillosis Pneumonia | Aspergillus fumigatus Pneumocystis jirovecii (carinii) |
Table 4.2 Examples of economically important fungi
| Fungal species | Application |
| Filamentous fungi | |
| Agaricus bisporus | Edible mushroom |
| Aspergillus, Penicillium spp. | Enzymes (catalase, lipase,amylase) |
| Aspergillus sp. + | Sake (rice wine) |
| Saccharomyces sp. | |
| Fusarium graminearum | Single cell protein |
| Penicillium chrysogenum | Penicillin production |
| Penicillium notatum | Enzyme (glucose oxidase) |
| Penicillium roqueforti | Cheese flavouring(Roqueforti ‘blue ’ cheese) |
| Yeast | |
| Pichia sp. | Gene expression system |
| Saccharomyces cerevisiae | Bakers ’ yeast—bread |
| Brewers ’ yeast—beer,wine, cider, etc. | |
| Enzyme (invertase) | |
| Gene expression system | |
| Dietary supplement |
Fungal taxonomy is extremely difficult and there is much discussion as to the number and inter-relatedness of fungal classes. Using one classification system, the kingdom Fungi can be subdivided into six classes. The class Oomycetes contains the mildews and water moulds, the class Ascomycetes contains the mildews, some moulds and most yeast species (including Saccharomyces cerevisiae), the class Basidiomycetes contains the mushrooms and bracket fungi, the class Teliomycetes contains the rust fungi (plant pathogens), the class Ustomycetes contains the smuts (plant pathogens) and the class Deuteromycetes contains species such as Aspergillus, Fusarium and Penicillium (see Figure 4.1). In contrast, phylogenetic analysis has revealed that there are four distinct phylawithin the fungal kingdom; these are the Chytridiomycota, Zygomycota, Ascomycota and Basidiomycota.
2 Structure of the fungal cell
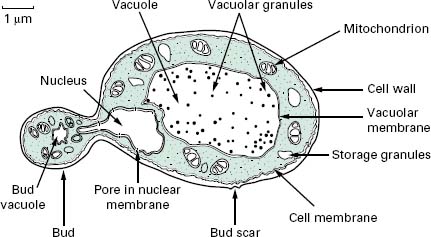
The typical yeast cell is oval in shape and is surrounded by a rigid cell wall which contains a number of structural polysaccharides and may account for up to 25% of the dry weight of the cell wall (see Figure 4.2). Glucan accounts for 50-60%, mannan for 15-23% and chitin for 1-9% of the dry weight of the wall, respectively, with protein and lipids also present in smaller amounts. The thickness of the cell wall may vary during the life of the cell but the average thickness in the yeast C. albicans varies from 100 to 300 nm. Glucan, the main structural component of the fungal cell wall, is a branched polymer of glucose which exists in three forms in the cell: β-1,6-glucan, β-1,3-glucan and β-1,3,-β-1,6-complexed with chitin. Mannan is a polymer of the sugar mannose and is found in the outer layers of the cell wall. The third principal structural component, chitin, is concentrated in bud scars that are areas of the cell from which a bud has detached. Proteins and lipids are also present in the cell wall and under some conditions may represent up to 30% of the cell wall contents. Mannoproteins form a fibrillar layer that radiates from an internal skeletal layer that is formed by the polysaccharide component of the cell wall. The innermost layer is rich in glucan and chitin which provides rigidity to the wall and is important in regulating cell division.
Enzymatic or mechanical removal of the cell wall leaves an osmotically fragile protoplast which will burst if not maintained in an osmotically stabilized environment. Incubation of protoplasts in an osmotically stabilized agar growth medium will allow the resynthesis of the wall and the resumption of normal cellular functions. The ability to generate fungal protoplasts opens the possibility of fusing these under defined conditions to generate strains with novel biotechnological applications.
The periplasmic space is a thin region that lies directly below the cell wall. It contains secreted proteins that do not penetrate the cell wall and is the location for a number of enzymes required for processing nutrients prior to entry into the cell. The cell membrane or plas-malemma is located directly below the periplasmic space and is a phopholipid bilayer which contains phospholipids, lipids, protein and sterols. The plasmalemma is approximately 10 nm thick and in addition to being composed of phospholipids also contains globular proteins. The dominant sterol in fungal cell membranes is ergosterol which is the target of the antifungal agent amphotericin B. Sterols are important components of the plasmalemma and represent regions of rigidity in the fluidity provided by the phospholipid bilayer.
Most of the cell’s genome is concentrated in the nucleus which is surrounded by a nuclear membrane which contains pores to allow communication with the rest of the cell (see Figure 4.2). The nucleus is a discrete organelle and, in addition to being the repository of the DNA, also contains proteins in the form of histones. Yeast chromosomes vary in size from 0.2 to 6 Mb and the number per yeast is also variable with S. cerevisiae having as many as 16 while the fission yeast Sch. pombe has as few as 3. In addition to the genetic material in the nucleus the yeast cell often has extrachromosomal information in the form of plasmids. For example, the 2 μ m plasmid is present in S. cerevisiae, although its function is unclear, and there are killer plasmids in the yeast Kluyveromyces lactis which encode a toxin.
Actively respiring fungal cells possess a distinct mitochondrion which has been described as the ‘powerhouse’ of the cell (Figure 4.2). The enzymes of the tricarboxylic acid cycle (Krebs’cycle) are located in the matrix of the mitochondrion while electron transport and oxidative phosphorylation occur in the mitochondrial inner membrane. The outer membrane contains enzymes involved in lipid biosynthesis. The mitochondrion is a semiindependent organelle as it possesses its own DNA and is capable of producing its own proteins on its own ribosomes which are referred to as mitoribosomes.
The fungal cell contains a vast number of ribosomes which are usually present in the form of polysomes— lines of ribosomes strung together by a strand of mRNA. Ribosomes are the site of protein biosynthesis. The system which mediates the export of proteins from the cell involves a number of membranous compartments including the Golgi apparatus, the endoplasmic reticulum and the plasmalemma. In addition, the vacuole is employed as a ‘storage space’ where nutrients, hydrolytic enzymes or metabolic intermediates are retained until required.
3 Medical significance of fungi
Fungi represent a significant group of pathogens capable of causing a range of diseases in humans under the right set of conditions. Although the majority of fungi appear to be harmless to humans it is worth bearing in mind that a normally nonpathogenic fungus can cause a clinically relevant problem if the immune system is suppressed as a result of therapy (e.g. for receipt of organ transplant) or disease (e.g. cancer). In a case of profound immuno-compromise a wide range of fungi can present as capable of inducing disease.
The most common fungal pathogens of humans can be divided into three broad classes: yeasts, moulds and dermatophytes. The yeast C. albicans is the most frequently encountered human fungal pathogen, being responsible for a wide range of superficial and systemic infections. The superficial infections include oropharyngeal and genital conditions, the former occurs predominantly in HIV positive individuals, geriatric patients and premature infants and may arise when a weakened or immature immune system is present. Genital candidosis is very common and approximately 75% of women are affected by vulvovaginal candidosis (VVC) during their life with a further 5-12% suffering from recurring bouts of infection over a prolonged period of time.
The mould Aspergillus fumigatus is the dominant fungal pulmonary pathogen of humans and generally presents as a problem in those with preexisting lung disease or damage. In addition to pulmonary infection other sites may be affected including the brain, kidneys and sinuses depending upon the level of immunocompromise of the individual. Groups particularly susceptible to colonization by Aspergillus species include those with cavities due to tuberculosis, patients affected with asthma or cystic fibrosis and those with profound immunosuppression due to leukaemia (neutropenia). Aspergillosis presents as a serious problem in patients immunosuppressed in advance of organ transplantation.
Dermatophyte is the term applied to a range of fungi capable of colonizing the skin, nails or hair. The principal dermatophytic fungi are Trichophyton, Microsporum and Epidermophyton species. The most commonly encountered dermatophytic infections are athlete’s foot (infection of the foot) and ringworm (fungal infection of the scalp or skin).
The choice and dose of an antifungal will depend upon the nature of the condition, whether there are any underlying diseases, the health of the patient and whether anti-fungal resistance has been identified as compromising therapy. Part of the difficulty in designing effective antifungal agents lies in the fact that fungi are eukaryotic organisms so agents that will kill fungi may also have a deleterious effect on human tissue. The ideal antifungal drug should target a pathway or process specific to the fungal cell, so reducing the possibility of damaging tissue and inducing unwanted side effects.
4.1 Polyene a ntifungals
Polyene antifungals are characterized by having a large macrolide ring of carbon atoms closed by the formation of an internal ester or lactone (Figure 4.3). In addition,polyenes have a large number of hydroxyl groups distributed along the macrolide ring on alternate carbon atoms. This combination of highly polar and non-polar regions within the molecule renders the polyenes amphiphatic, i.e. having hydrophobic and hydrophilic regions in the one molecule, which assists solubility in lipid membranes.
Figure 4.3 Structures of polyene (amphotericin B) and azole (itraconazole, fluconazole, miconazole and ketoconazole) antifungal agents.
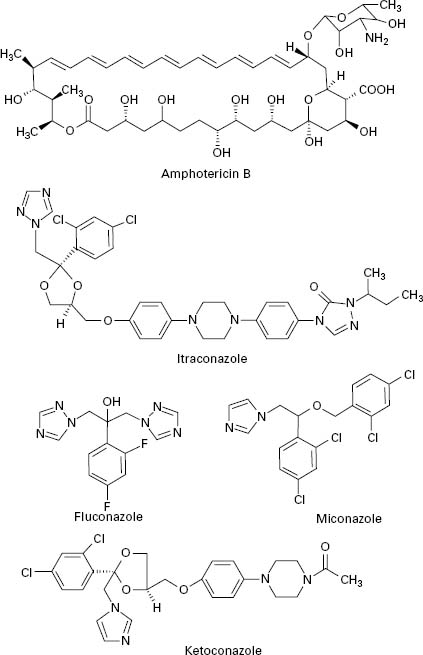
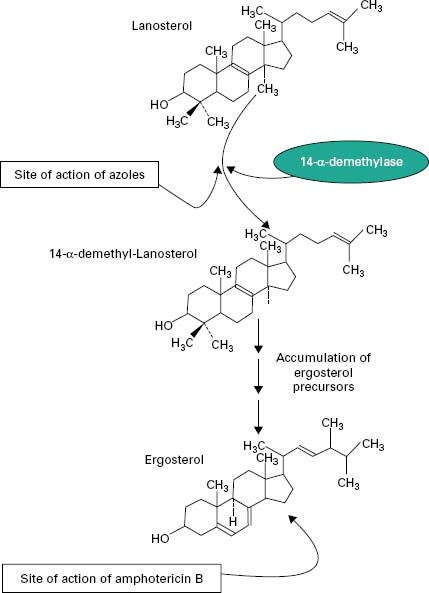
The principal polyenes are amphotericin B and nystatin. Amphotericin B is produced by the bacterium Streptomyces nodosus and its activity is due to the ability to bind ergosterol, which is the dominant sterol in fungal cell membranes, and consequently increases membrane permeability by the formation of pores (Figure 4.4). The action of amphotericin B seems to rely on the formation of pores through which intracellular contents can escape from the cell. Amphotericin B can lead to renal damage during prolonged antifungal therapy. Amphotericin B is active against a broad range of fungal pathogens and is considered the ‘gold standard’ against which the activity of other antifungal agents is measured. Because of its renal toxicity amphotericin B tends to be reserved for severe cases of systemic fungal disease but recent formulations in which the drug is encapsulated within liposomes have been shown to have reduced toxicity.
Nystatin was discovered in 1950 and exhibits the same mode of action as amphotericin B but tends to have lower solubility, which has restricted its use to the treatment of topical infections. Although nystatin was effective for the treatment of conditions such as oral and vaginal candidosis, its use has been overtaken by the introduction of azole antifungal drugs.
4.2 Azole a ntifungals
The first generation of azole antifungals revolutionized the treatment of mucosal and invasive fungal infections, and azoles are still the most widely used group of antifungal agents. The azole derivatives are classified as imidazoles or triazoles on the basis of whether they have two or three nitrogen atoms in the five-membered azole ring (Figure 4.3). The azoles in current clinical use are clotrimazole, miconazole, econazole and ketoconazole; newer drugs such as itraconazole, fluconazole and voriconazole have important applications in the treatment of systemic infections. Azoles function by interfering with ergosterol biosynthesis by binding to the cytochrome P-450 mediated enzyme known as 14-α-demethylase (P-450DM).This blocks the formation of ergosterol by preventing the methylation of lanosterol (a precursor of ergosterol) (Figure 4.4). This result in a reduction in the amount of ergosterol in the fungal cell membrane which leads to membrane instability, growth inhibition and cell death. An additional consequence of the block in ergosterol biosynthesis is the build up of toxic intermediates which can prove fatal to the fungal cell.
Azoles exhibit a broad spectrum of activity in vitro,being capable of inhibiting the growth of most Candida, Cryptococcus and Aspergillus species, and dermatophytes. Miconazole was the first azole used to treat systemic fungal infections but demonstrated a number of toxic side effects. Ketoconazole produced high serum concentrations upon oral administration but had poor activity against aspergillosis. In addition, ketoconazole was associated with a range of side effects which limited its applicability. Newer triazoles such as fluconazole and itraconazole have increased the options for dealing with fungal infections. Fluconazole was introduced for clinical use in 1990, is water soluble and shows good penetration and deposition into the pulmonary tissues; it also reaches high levels in the cerebrospinal fluid and the peritoneal fluids. Fluconazole has proved highly effective in the treatment of infections caused by C. albicans but shows limited activity against Aspergillus. Itraconazole became available for clinical use in the late 1980s and was the first azole with proven efficacy against Aspergillus.Itraconazole is effective in treating severe Aspergillus infections and exhibits both fungicidal and fungistatic effects. Upon ingestion itraconazole undergoes extensive hepatic metabolism which yields up to 30 metabolites, a number of which retain antifungal activity. Itraconazole is currently available as an intravenous formulation and is widely used for the treatment of severe Aspergillus infection in this form. Fluconazole and itraconazole demon0strate significantly reduced side effects compared to ketoconazole. Novel azole drugs with increased ability to inhibit the fungal 14-α demethylase are also becoming available. These agents, which include voriconazole, posaconazole and ravuconazole, have a wider spectrum of activity than fluconazole and it has been suggested that some of them show fungicidal effects to some species (e.g. Aspergillus spp.). Voriconazole is one of the newest second generation triazole antifungal drugs and it shows good activity against pulmonary aspergillosis and cerebral aspergillosis.
4.3 Echinocandins
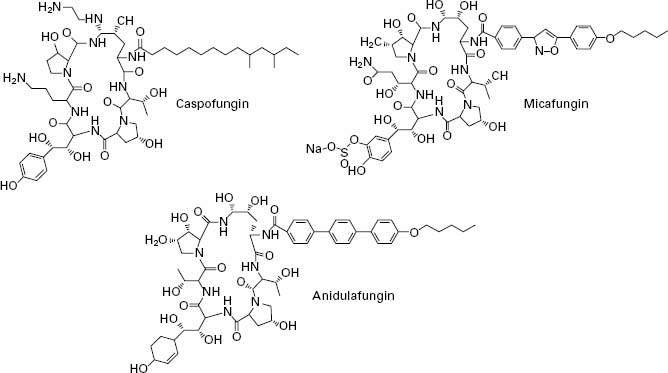
The echinocandins are a relatively new group of antifungal drug and are semisynthetic lipopeptides comprising a cyclic hexapeptide core connected to a lateral fatty acid chain. Three compounds of this group are currently in use: caspofungin, micafungin and anidulafungin (Figure 4.5). Unlike conventional antifungal therapy that targets ergosterol or its synthesis, the echinocandins target the synthesis of β 1,3-glucan, the major polymer of the fungal cell wall. The cell wall is essential to the fungus as it provides physical protection, maintains osmotic stabil-ity, regulates cell shape, acts as a scaffold for proteins, mediates cell cell communication and is the site of a number of enzymatic reactions. Inhibition of β-1,3-glucan synthesis disrupts the structure of the growing cell wall, resulting in osmotic instability and ballooning out of the intracellular contents as a result of high osmotic pressure, and ultimately ends in cell lysis.
< div class='tao-gold-member'>