First and Second Trimester Pregnancy Loss
Jennifer Pogoriler, M.D., Ph.D.
Theonia K. Boyd, M.D.*
*Based upon the prior edition chapter by Deborah E. McFadden
Pathologic examination of the products of embryos and fetuses, both from spontaneous abortions (SAbs) and terminations of pregnancy, has become increasingly important. While such examination was once performed primarily for the purpose of furthering scientific understanding of prenatal human development, the practical medical applications of this knowledge have become clear and now form an integral part of the medical assessment and management of fertility issues (1,2,3,4,5). As an understanding of the factors involved in successful pregnancies has developed and as patient demand for information has increased, the role of pathologic examination has grown. Increased use of assisted fertilization techniques has heightened the interest of physicians and patients alike in understanding why pregnancies fail. This chapter will address the examination of disorders encountered in those pregnancies that end spontaneously in the first and second trimesters of gestation; the pathology of fetuses delivered after pregnancy termination after prenatal ultrasound diagnosis is beyond the scope of this chapter.
It is recognized that many conceptions do not end in live births but, rather, that there is a high rate of spontaneous loss, especially early in gestation. It is estimated that 10% to 20% of recognized pregnancies end as SAbs, with most losses occurring in the first trimester (first 12 to 14 weeks of gestation). With the demonstration of fetal cardiac activity, the miscarriage rate drops to approximately 3% to 12% (6). In a study of women with a normal prenatal visit at 6 to 11 weeks of gestational age (GA), the risk of subsequent SAb was 1.6% or less, considerably lower than for pregnancies overall (7). After the first trimester, approximately 1% to 2% of pregnancies are spontaneously aborted (8). The incidence of stillbirth at term gestation is in the order of 0.1% to 0.5%. These loss rates that persist throughout gestation, together with changing or changed societal approaches and expectations of pregnancy such as delaying childbearing until later in a woman’s reproductive life and increased access to assisted reproduction methods, have led to an intense interest in understanding the cause of pregnancy loss and the implications for future reproductive success.
GA refers to the number of weeks since the last menstrual period (equivalent to menstrual dates), while developmental age (DA) or conceptual age (CA) refers to the age as determined from the time of fertilization, generally considered as approximately 2 weeks after the last menstrual period. Embryos are assessed by developmental features that correlate with age, usually given as DA. Thus, in a normal gestation, GA is DA plus 2 weeks.
The first trimester of pregnancy is the period of implantation and embryogenesis, with the completion of embryogenesis by 8 weeks of DA (10 weeks of GA). Upon completion of embryogenesis with development of all organ systems, the conceptus is referred to as a fetus. Definitions of fetus and infant vary with locale; in Canada, a fetus is considered an infant once it has reached the GA of 20 weeks or is liveborn at any GA. In the United States, by contrast, the living intrauterine conceptus is referred to as a fetus until the time of live birth. Stillbirth is defined as delivery of a deceased conceptus at or after 20 weeks of GA.
CAUSES OF EARLY SPONTANEOUS ABORTION
It is well recognized that the major causes of early SAb are lethal chromosomal abnormalities, which are usually aneuploid (any karyotype that deviates from two haploid sets of 22 autosomes and one sex chromosome, with each haploid set contributed by one parent) (9). The use of techniques such as comparative genomic hybridization (CGH) and quantitative fluorescence-polymerase chain reaction (QF-PCR) to supplement conventional cytogenetic analysis has increased the detection of chromosome abnormalities because these
techniques do not require cell culture and can address the issue of maternal cell contamination. Numerous studies have shown that at least half of early SAbs are chromosomally abnormal when routine cytogenetics is performed. Array CGH may be used in cases in which tissue culture for cytogenetic analysis has failed or in which maternal cell contamination is suspected (10,11,12). More recent studies of preimplantation blastomeres in in vitro fertilization (IVF) patients have shown that even in young healthy couples, fewer than 10% of embryos have a normal karyotype in all blastomeres (13), although it is unclear if this is due to IVF or reflects a typical state of chromosomal instability in early embryonic life.
techniques do not require cell culture and can address the issue of maternal cell contamination. Numerous studies have shown that at least half of early SAbs are chromosomally abnormal when routine cytogenetics is performed. Array CGH may be used in cases in which tissue culture for cytogenetic analysis has failed or in which maternal cell contamination is suspected (10,11,12). More recent studies of preimplantation blastomeres in in vitro fertilization (IVF) patients have shown that even in young healthy couples, fewer than 10% of embryos have a normal karyotype in all blastomeres (13), although it is unclear if this is due to IVF or reflects a typical state of chromosomal instability in early embryonic life.
The distribution of chromosome abnormalities is consistent between various studies, with trisomy accounting for half of all chromosomally abnormal SAbs, polyploidies and monoploidies (primarily monosomy X) each accounting for 10% to 15%, and structural rearrangements accounting for approximately 5%. (14,15). Trisomy for two chromosomes (double trisomy) is seen in only 3% of the chromosomally abnormal early SAbs. Trisomies for chromosomes 16, 21, and 22 are most common in early SAbs. The structural abnormalities are an important subgroup because of the possibility that they have arisen from a parent who carries a rearrangement predisposing to an unbalanced karyotype in the offspring. Studies of couples who have had recurrent miscarriages show that 5% have balanced rearrangements such as reciprocal translocations (16). These individuals have an increased risk of conceiving chromosomally abnormal pregnancies as well as recurrent miscarriage (17), with the actual risk depending on the type of rearrangement and the chromosomes involved (18). Although the presence of a balanced translocation predicts a risk of increased miscarriage in future pregnancies, the risk of a liveborn infant with a translocation is extremely low, and the proposed benefit of preimplantation genetic diagnosis is to shorten the interval between pregnancies, in order to achieve a successful outcome (19) rather than to improve the overall live birth rate.
With respect to trisomic conception, the most common subset of nonviable aneuploidies, studies of parental origin have demonstrated that the largest proportion of trisomy is maternally derived. There is a strong positive correlation with maternal age, meaning that the risk of conceiving a trisomic conceptus rises with increasing maternal age (20). Hypotheses to explain this finding include abnormal chromosomal crossover, altered chromatin cohesion, and altered DNA damage checkpoints. Trisomy affects 3% of pregnancies in 25-year-old women but occurs in 35% of pregnancies in women aged 42 years (21). Most trisomies are the result of errors in meiosis I (22), although this varies for individual chromosomes. For example, trisomy 18 is more typically the result of errors in the second meiotic division, while trisomy 21 is predominantly the result of first meiotic division errors.
With the predominance of SAbs attributable to aneuploidy, it is clear that in order to ensure clinical relevance of SAb evaluations, the examination should include determination of karyotype, particularly in recurrent pregnancy loss. In the event that the examination proves normal, with normal karyotype and normal villus histology, management of persons having recurrent SAbs shifts and other etiologies for pregnancy loss must be considered (23). The investigation of those who have had chromosomally normal miscarriages with no other pathology is dependent upon the proposed nonchromosomal mechanisms of pregnancy loss. These proposed associations include exogenous environmental exposures, uterine abnormalities (24), skewed X-inactivation (25,26), disorders of endocrine function, immune disorders including conditions with autoantibodies, and thrombophilic conditions (15). The roles of these factors in miscarriage remain under investigation, and the relative significance of each has not been established with certainty. Prospective cohort studies have not shown an association between single inherited thrombophilia mutations and miscarriage, although multiple mutations may increase risk (27,28,29,30). Prophylactic anticoagulant treatment has not been shown to improve outcomes in patients with recurrent miscarriages and inherited thrombophilias, and screening for these conditions is therefore not currently recommended (31), although this practice remains controversial (30). There is less controversy about the role of antiphospholipid antibody in recurrent pregnancy loss. Antiphospholipid antibodies are found more often in women who have recurrent miscarriages (RSAb) than in other women, although the mechanism by which an antiphospholipid antibody causes pregnancy loss is not definitively known. It is speculated that it may be due to interference with trophoblast function and thus the establishment of successful implantation, mediated by early-onset inadequate decidual arteriolar perfusion, and/or due to maternal/gestational interface inflammation (32). There are no specific pathologic features identified in the first trimester SAb from women who are positive for this antibody. Later findings seen in a subset of patients are also nonspecific but include those changes seen in other causes of uteroplacental malperfusion including infarction, increased intervillous fibrin, and terminal villous hypoplasia (33,34,35). Current guidelines recommend screening in patients with prior venous thromboembolism, with one fetal loss or with multiple embryonic losses. Treatment with prophylactic anticoagulation may improve outcome in these patients (36). With changing reproductive patterns, such as women starting their families later in life and having fewer children, there is enhanced societal desire to diagnose and manage causes of miscarriage. Given that cytogenetic abnormalities account for the majority of first trimester abortions, it has been suggested that the evaluation of first trimester SAb with cytogenetic analysis may prove more cost effective than a standard battery of tests such as thyroid function tests, endometrial biopsy, or thrombophilia testing (37). Pathologic examination also serves to identify those conditions not associated with abnormal karyotype that may require additional investigation or treatment to increase the likelihood of successful
pregnancy and to diagnose conditions in which there is a risk of neoplasia, as with molar gestations and their attendant risk of transformation to gestational trophoblastic neoplasia (GTN) (1).
pregnancy and to diagnose conditions in which there is a risk of neoplasia, as with molar gestations and their attendant risk of transformation to gestational trophoblastic neoplasia (GTN) (1).
FIRST TRIMESTER SPONTANEOUS ABORTION
Indication for Cytogenetic Analysis
Given that chromosome abnormality accounts for the majority of first trimester SAbs, an argument could be made for performing cytogenetic analysis on all cases. To assist in the reproductive counseling regarding cause of the SAb and risks for recurrence, karyotype is a vital piece of information. Where embryo-pathology examination was once performed only in cases of recurrent SAbs, changes in reproductive patterns and practices have altered, resulting in a broader range of cases referred for embryo-pathologic examination. Those who treat women who have had difficulty conceiving or who have had previous miscarriage(s) are anxious to know whether the pregnancy failure was the result of chromosome abnormality or if there is perhaps another etiology necessitating further investigation. Increasingly, assisted reproductive technologies (ARTs) such as IVF or intracytoplasmic sperm injection (ICSI) are utilized. There are concerns that ARTs are associated with an increased incidence of chromosome abnormalities at prenatal diagnosis and at birth, specifically for sex chromosome abnormalities in pregnancies that are the result of ICSI (38,39). However, many small studies have found a high rate of chromosomal abnormalities in both ART and naturally conceived miscarriages, and a recent meta-analysis of case-controlled studies of chromosomal abnormalities in first trimester miscarriages showed no significant differences between either IVF or ICSI and natural conception, with the risk of abnormalities increased in older women in all groups (40).
Until the natural history of ART pregnancies is delineated, the use of these technologies should be considered as an indication for cytogenetic analysis in cases of SAb. In some laboratories, the fact that the majority of first trimester SAbs are the result of chromosome abnormality is sufficient indication to perform cytogenetic analysis of all cases examined morphologically. In other laboratories, constraints imposed by funding structures may impose the necessity of specific clinical indication before cytogenetic studies will be funded. These other indications include a history of recurrent miscarriages (variably defined as two to three or more losses), abnormal villus morphology, abnormal or normal embryo, parental chromosome rearrangement, and maternal age 35 years or greater.
Examination
Examination of the early pregnancy loss or embryo specimen is quite different from that of a fetal specimen as the latter represents an autopsy examination of a fetus and its placenta. Examination of the products of an early pregnancy loss (spontaneous or missed abortion) is performed to identify pregnancy-related tissues (embryo and/or placental tissue) to confirm intrauterine pregnancy and to assess their morphology. This examination includes sampling of tissues for additional studies, including for cytogenetic analysis or other means of determining the chromosome complement of the conceptus. Thus, it is imperative that all specimens for embryo-pathology examination are submitted in the fresh rather than fixed state.
An assessment of the products of conception is best accomplished by examining the specimen under a dissecting microscope, ideally equipped with a camera. The presence of any embryonic or placental tissue, including implantation site, allows confirmation of intrauterine pregnancy. In the absence of pregnancy-related tissues, intrauterine pregnancy cannot be confirmed, and the report must reflect that. Decidualized endometrium may be seen in estrogen effect, including with ectopic pregnancy, and is therefore insufficient for confirmation of intrauterine pregnancy.
The morphology of the chorionic villi is characterized— their individual morphology and their distribution over the chorionic sac. Attention to whether the villi appear overly abundant and/or cystic is important because of concerns for complete or partial hydatidiform mole (CHM, PHM). Other features of embryonic development, such as amnion, yolk sac, umbilical cord, and nucleated embryofetal erythrocytes, should also be assessed.
Tissues are sampled for cytogenetic analysis and can be retained frozen for additional studies as required. In many institutions, tissue is often submitted directly to the cytogenetics laboratory by the clinicians, either from the tissue obtained at dilation and curettage or by chorionic villous sampling. Additional tissue may be retained frozen for array CGH, although this can be increasingly performed on paraffin-embedded tissue (41). Although an argument can be made to utilize array CGH in all cases of SAbs to eliminate the labor and risk of culture failure associated with conventional cytogenetic analysis, conversely, CGH will miss some anomalies seen only on karyotype (12,42,43,44).
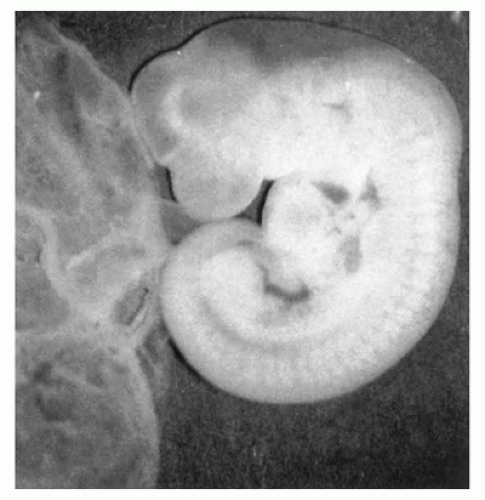
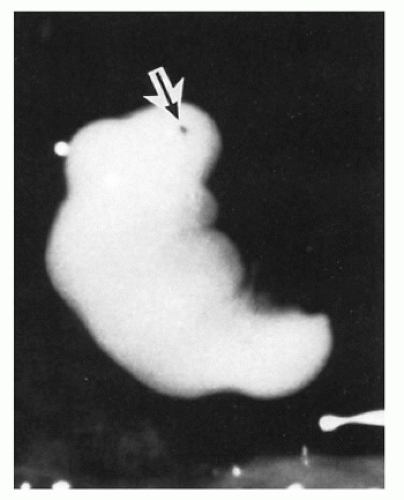
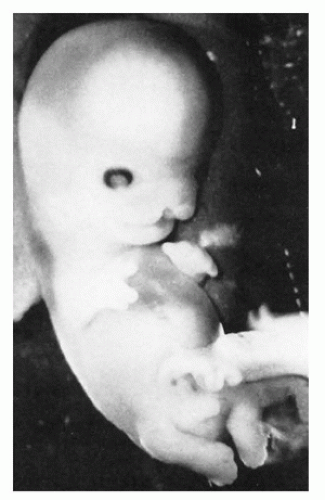
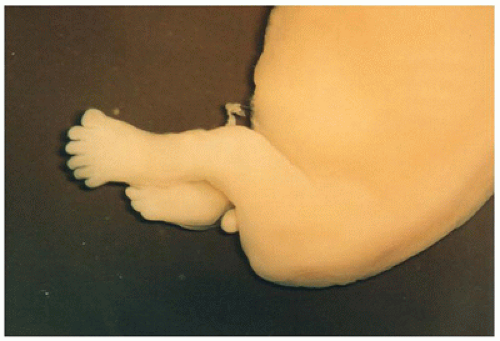
The presence of embryonic tissue confirms intrauterine pregnancy and is a feature in favor of a diagnosis other than CHM, a frequent concern as edematous villi are often identified on ultrasound or at gross examination. In determining the developmental stage and thus age of the embryo, standard developmental criteria can be used (45,46). Embryos may be normally developed (Figure 2-1) according to established criteria, but this does not exclude chromosome abnormality. Most developmental tables were established without karyotype determination. Some cases do not show regularly developed embryos but rather embryos or embryonic tissues in which normal developmental features are not present, a state referred to as growth disorganization. Growth disorganization has been divided into four categories: type I growth-disorganized embryo (GD1)
refers to an intact empty sac (Figure 2-2), type II refers to a nodular embryo in which cranial and caudal ends cannot be distinguished (Figure 2-3), type III refers to a cylindrical embryo in which there is some cranial-caudal differentiation with retinal pigment (Figure 2-4), and type IV refers to an embryo in which there is more recognizable embryonic development but delayed growth of limbs and other developmental features (Figure 2-5). While growth disorganization is readily identified and classified, the findings are nonspecific—in all types of growth-disorganized embryos, the incidence of chromosome abnormality is similar to that encountered in SAbs in general, with the same types of chromosome abnormalities identified. Ultrasound detection of an embryo does not strictly correlate with the morphologic detection of embryonic tissue, but it has been demonstrated that the rates of abnormal karyotypes are not significantly different between SAbs in which an embryonic pole is identified on ultrasound examination and those that appear anembryonic (37,47). Approximately 60% to 70% of both anembryonic and embryonic specimens are chromosomally abnormal, although the spectrum of abnormalities may be different, with fewer monosomy X and viable autosomal trisomies in the anembryonic specimens.
refers to an intact empty sac (Figure 2-2), type II refers to a nodular embryo in which cranial and caudal ends cannot be distinguished (Figure 2-3), type III refers to a cylindrical embryo in which there is some cranial-caudal differentiation with retinal pigment (Figure 2-4), and type IV refers to an embryo in which there is more recognizable embryonic development but delayed growth of limbs and other developmental features (Figure 2-5). While growth disorganization is readily identified and classified, the findings are nonspecific—in all types of growth-disorganized embryos, the incidence of chromosome abnormality is similar to that encountered in SAbs in general, with the same types of chromosome abnormalities identified. Ultrasound detection of an embryo does not strictly correlate with the morphologic detection of embryonic tissue, but it has been demonstrated that the rates of abnormal karyotypes are not significantly different between SAbs in which an embryonic pole is identified on ultrasound examination and those that appear anembryonic (37,47). Approximately 60% to 70% of both anembryonic and embryonic specimens are chromosomally abnormal, although the spectrum of abnormalities may be different, with fewer monosomy X and viable autosomal trisomies in the anembryonic specimens.
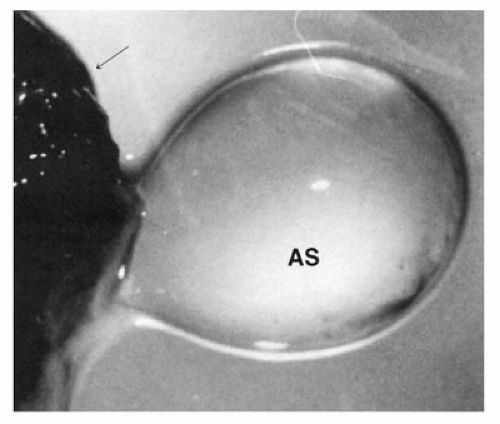 FIGURE 2-2 • Growth-disorganized embryo, GD1—intact empty amniotic sac (AS). Opened chorionic sac (arrow). |
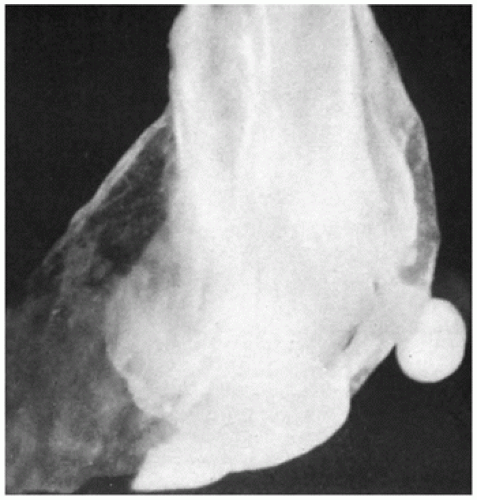 FIGURE 2-3 • Growth-disorganized embryo, GD2—1 mm nodular embryo in opened amniotic and chorionic sac. |
Embryos may show isolated or focal abnormalities such as neural tube defects, facial clefts, or limb anomalies
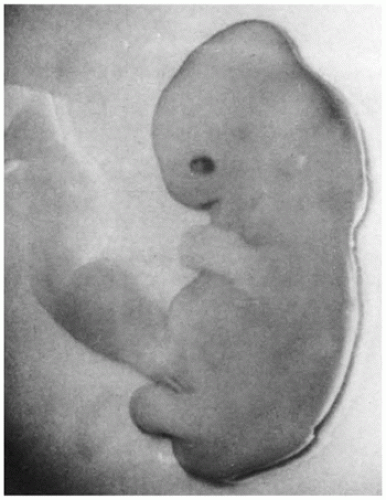
(Figures 2-6, 2-7, 2-8). Many of these abnormalities, such as neural tube defects, occur in the setting of chromosome abnormality (48). In the setting of a normal karyotype, the focal defects likely have the same significance as in later gestation, and genetic counseling to discuss the findings and possible recurrence risks is indicated. Chromosomally abnormal embryos may show a number of nonspecific abnormalities, such as delay of normal limb development and various types of growth disorganizations. Embryos with trisomies more commonly encountered in later gestation and live births, such as trisomies 13, 18, and 21, may show some features in common with the phenotypes
observed in the fetal period. Most often, however, embryonic phenotypic manifestations of these trisomies are nonspecific (Figure 2-9).
(Figures 2-6, 2-7, 2-8). Many of these abnormalities, such as neural tube defects, occur in the setting of chromosome abnormality (48). In the setting of a normal karyotype, the focal defects likely have the same significance as in later gestation, and genetic counseling to discuss the findings and possible recurrence risks is indicated. Chromosomally abnormal embryos may show a number of nonspecific abnormalities, such as delay of normal limb development and various types of growth disorganizations. Embryos with trisomies more commonly encountered in later gestation and live births, such as trisomies 13, 18, and 21, may show some features in common with the phenotypes
observed in the fetal period. Most often, however, embryonic phenotypic manifestations of these trisomies are nonspecific (Figure 2-9).
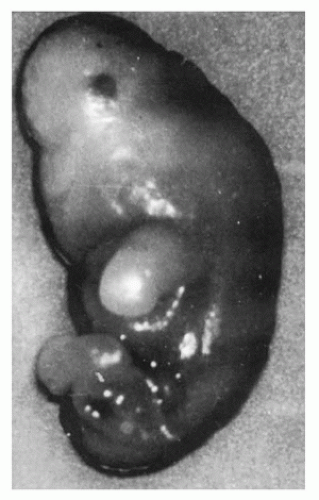 FIGURE 2-5 • Growth-disorganized embryo, GD4—delayed development of head, trunk, and limbs relative to crown-rump length. |
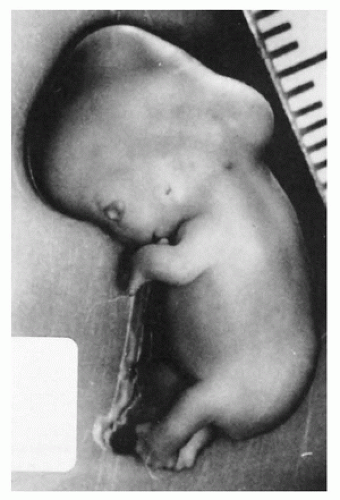
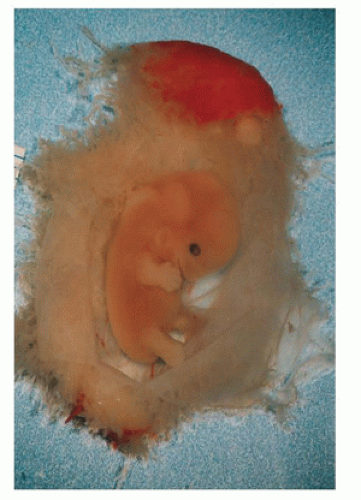
Triploidy is encountered in approximately 6% of early SAbs; embryonic tissues are often identified. A phenotype thought to be characteristic of embryonic triploidy has been described and includes retarded limb development, facial dysplasia, subectodermal hemorrhage, and cystic chorionic villi (49) (Figure 2-10), but more recent series describe a wide range of phenotypic abnormalities ranging from growth-disorganized to apparently normal embryos (50). These apparently normal embryos are most often seen at approximately stage 16 of development (37 to 42 days), equivalent to approximately 7 to 8 weeks of GA (Figure 2-11). The normal and the growth-disorganized phenotypes have been seen in triploids of both maternal origin and paternal origin.
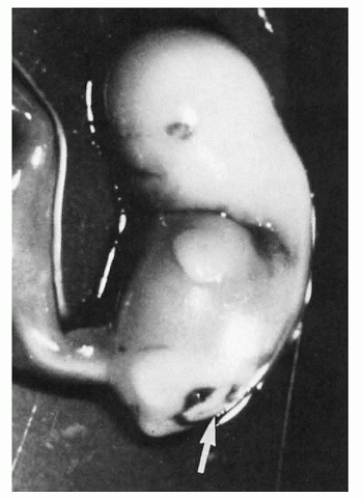 FIGURE 2-10 • Triploid embryo showing dysplastic face, delayed limb development, and defect in lumbosacral region (arrow). |
Histologic examination of placental tissues and decidua should be routinely performed in all cases. Microscopic examination of chorionic villi allows detection of many potential abnormalities including viral infections such as cytomegalovirus (CMV) and bacterial infections such as Listeriosis. In addition, other disorders such as intervillositis or conditions with increased intervillous fibrin are occasionally detected. Villous infarction is distinctly unusual and should raise concerns of maternal vascular/thrombophilic disease. Routine histologic examination of decidua allows for assessment of decidual (maternal) vasculature. In a review of the histopathology of SAbs with known karyotype, 19% of SAbs with a normal karyotype showed evidence of chronic inflammation or perivillous fibrin deposition, in contrast to 8% of those with an abnormal karyotype. The findings were even more frequent (31%) in the subset of SAbs that were chromosomally normal and occurred in a population with recurrent SAbs (51).
Chronic histiocytic intervillositis is a disorder in which there is an abnormal increase in maternal mononuclear cells, predominantly comprised of mature CD68 histiocytes, within the intervillous (maternal) space (Figure 2-12). The lesion is thought to be a maternal immune-mediated disorder; it confers a high recurrence risk in subsequent pregnancies (52,53,54).
Increased perivillous fibrin is another disorder in which it has been suggested that maternal immune dysregulation may play a role. Perivillous fibrin may also be increased as a degenerative change in response to prolonged intrauterine death, and distinguishing between pathologic and degenerative changes in perivillous fibrin can be difficult. When there is an obvious increase in perivillous fibrin, the lesion may be considered to account for
the loss. Some suggest that an arbitrary threshold of 50% villous involvement be used to make a pathologic diagnosis (55). Although probably etiologically heterogeneous, this entity may also recur and has been associated with recurrent SAbs.
the loss. Some suggest that an arbitrary threshold of 50% villous involvement be used to make a pathologic diagnosis (55). Although probably etiologically heterogeneous, this entity may also recur and has been associated with recurrent SAbs.
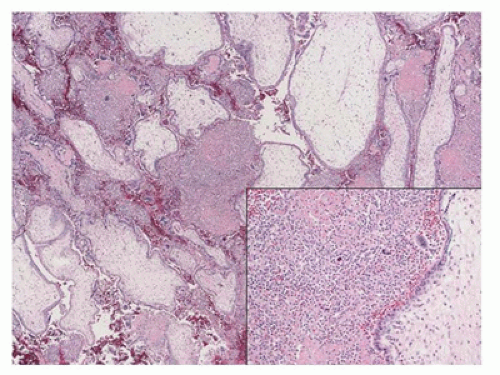 FIGURE 2-12 • Chronic (histiocytic) intervillositis in SAb at 10 weeks GA.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|