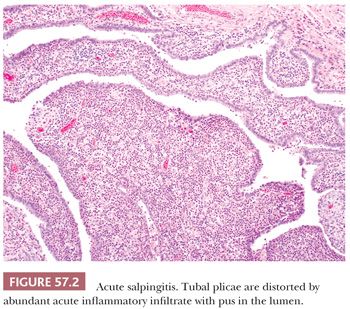
Neisseria gonorrhoeae and Chlamydia trachomatis are common initiating sexually transmitted pathogens, with 15% of endocervical infections resulting in pelvic inflammatory disease (PID) (1–3). There are conflicting reports regarding the role of other possible sexually transmitted initiating agents, such as bacterial vaginosis and various Mycoplasma species, in the development of PID (4–8). Regardless of the initiating pathogen, however, PID should be regarded and treated as a polymicrobial infection. A variety of organisms, including groups A and B Streptococci, Escherichia coli, Klebsiella species, Proteus mirabilis, Haemophilus species, Bacteroides/Prevotella species, Peptococcus, and Peptostreptococcus species, has been isolated, with Haemophilus influenzae and Streptococcus pyogenes associated with clinically severe disease (4,9–11). Mixed infection has been found in up to 50% of gonococcal PID (12).
Mild acute inflammation may be seen in the tubal lumen and lamina propria in “physiologic salpingitis” during the menstrual cycle, particularly at the time of menstruation or puerperium, and can be seen in fallopian tubes removed during postpartum ligation (13,14). In addition to the presence of acute inflammatory cells, edema and lymphatic dilation in the tubal plicae are often seen. The inflammation rarely involves the muscularis and lacks necrosis, mucosal ulceration, and subsequent chronic inflammation. This type of acute salpingitis appears to have no clinical significance.
CHRONIC SALPINGITIS
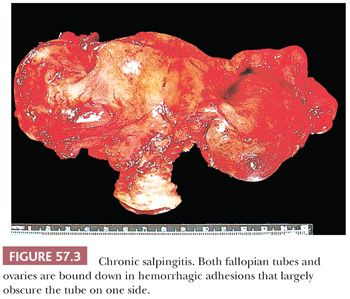
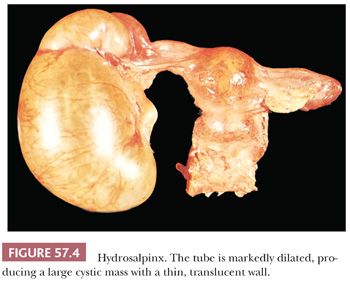
When acute salpingitis resolves, the acute and most of the chronic inflammation eventually subside, resulting in either complete resolution or chronic salpingitis with or without accompanying hydrosalpinx. Grossly, the fallopian tube in the latter sequela is enlarged, distorted, and often exhibits serosal and tubo-ovarian adhesions (Fig. 57.3). Coalescence of the fimbriae and occlusion of the ostium may lead to hydrosalpinx that is often bilateral. In such cases, the tube may appear cystic with a thin, translucent wall and contains serous or purulent fluid (Fig. 57.4).
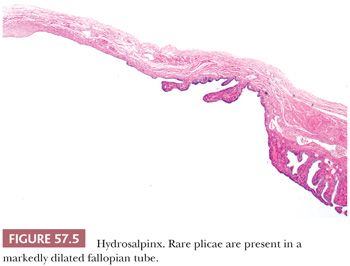
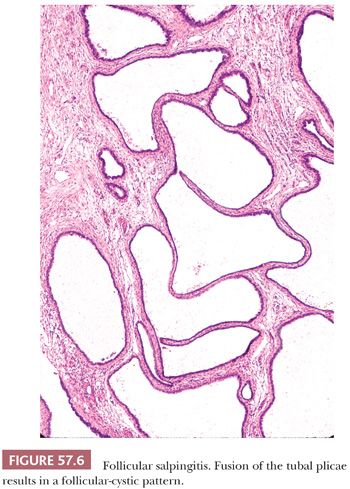
Microscopically, plicae are reduced in number, blunted, and fibrotic. Lymphocytes and plasma cells are present, although they may be sparse in the late stages of chronic salpingitis. The epithelium is often flattened to low cuboidal, but occasional plicae may demonstrate typical columnar tubal epithelium. Observation of rare plicae and an attenuated myosalpinx is helpful in distinguishing hydrosalpinx from an ovarian or paraovarian cyst or a cystadenoma (Fig. 57.5). Chronic inflammatory cells are usually absent in hydrosalpinx. Bridging of the plical folds can form follicle-like spaces, resulting in follicular salpingitis (Fig. 57.6).
GRANULOMATOUS SALPINGITIS
Tuberculous Salpingitis
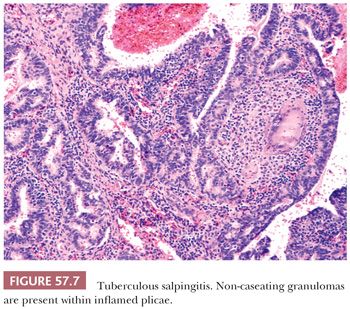
The most common etiology of granulomatous salpingitis is Mycobacterium tuberculosis or Mycobacterium bovis. Genital tuberculosis is rarely due to primary infection of the genitalia and is more frequently secondary to hematogenous spread from other sites of disease, particularly primary pulmonary infection. Genital tuberculosis is rare in Western countries but common in endemic regions worldwide, accounting for almost 50% of tubal causes of infertility in India (15–17). For unknown reasons, the fallopian tube is the most common female genital tract organ affected by tuberculosis, with approximately 90% of women with genital tuberculosis having tubal involvement (18). The infectious process is frequently bilateral (18). In early or mild infection, the fallopian tube is enlarged with a thickened or nodular wall. In more severe forms of the disease, dense serosal or tubo-ovarian adhesions, ulcerated mucosa, and/or caseous luminal exudate mimicking acute salpingitis may be seen. In contrast to most other forms of chronic salpingitis, the ostium usually remains patent.
Microscopically, granulomatous inflammation with multinucleated giant cells, epithelioid histiocytes, and lymphocytes is present (Fig. 57.7). Focal or massive caseation may be observed, and Schaumann bodies can be conspicuous. Granulomas typically involve the mucosa, but can extend into the myosalpinx and to the serosa. They may be sparse or absent, however, in immunosuppressed patients. The mucosal inflammatory reaction may lead to varying degrees of chronic inflammation, fibrosis, and calcification. Marked epithelial hyperplasia and distortion simulating adenocarcinoma can be seen. Stains for acid-fast organisms or mycobacterial culture should be performed when tuberculosis is suspected.
Actinomycotic Salpingitis
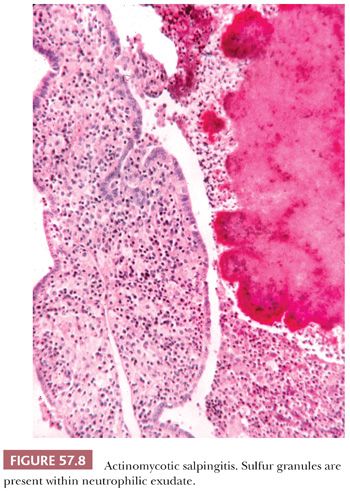
Actinomyces israelii is a rare cause of salpingitis and is found most often in association with use of an intrauterine device (19–22). Both tubes are frequently affected, and ovaries may also be involved. Yellow specks of sulfur granules within the luminal or serosal exudate may be seen grossly in addition to nonspecific manifestations of acute and chronic salpingitis. The presence of sulfur granules composed of colonies of Gram-positive, filamentous bacteria within a neutrophilic exudate is diagnostic (Fig. 57.8). Chronic inflammation, including histiocytes, is often seen, although a granulomatous reaction is rarely encountered.
Fungal Salpingitis
Fungi that may cause granulomatous salpingitis or tubo-ovarian abscesses include Blastomyces dermatitidis (23,24), Coccidioides immitis (25–30), Candida glabrata (31–33), and Aspergillus (34). Organisms are usually identifiable on microscopic examination. Inflammation may result from hematogenous spread, ascending infection, or disseminated disease.
Parasitic Salpingitis
Schistosomiasis of the fallopian tube is common in endemic regions worldwide, but rare in the United States. Schistosoma haematobium is the most common cause of granulomatous salpingitis, although Schistosoma mansoni may also be seen (35–37). A nodular and scarred fallopian tube is characteristic of tubal schistosomiasis and appears secondary to fibrosis elicited by the ova. Histologically, a granulomatous inflammatory reaction is observed. Ectopic pregnancy associated with tubal schistosomiasis has been reported (35,38–40).
Enterobius vermicularis may migrate up the lower female genital tract and infest the fallopian tube (41–45). A granulomatous reaction surrounding portions of a gravid female worm or ova may be seen. Oxyuriasis may also cause tubo-ovarian abscesses and generalized peritonitis (46).
Cysticercosis (Taenia solium) and amebiasis (Entamoeba histolytica) of the fallopian tube have been reported (47,48).
Sarcoidosis
Sarcoidosis may rarely involve the fallopian tube (49). Schaumann bodies can be present, although they may also be seen in Crohn disease and tuberculosis. Consideration of clinical history and exclusion of mycobacterial causes of granulomatous inflammation facilitate the diagnosis.
Crohn Disease
Crohn disease of the lower gastrointestinal tract may secondarily involve the fallopian tube to produce a granulomatous salpingitis, tubo-ovarian abscess, or tubo-intestinal fistula (50–54). Non-caseating granulomas may be present in the mucosa and muscularis. Like tuberculous salpingitis, a florid atypical hyperplasia of the tubal epithelium may be seen, mimicking carcinoma (51).
Foreign Body Salpingitis
Granulomatous salpingitis may arise secondary to irritants, including talc, starch, suture material, lubricant, or mineral oil (55–57). Pulse granulomas can also occur in the fallopian tube (58). Embolization agents, including trisacryl gelatin microspheres, polyvinyl alcohol particles, and polyvinyl alcohol microspheres, used in uterine artery embolization of leiomyomata can be present in non-targeted sites, including the fallopian tube (59). Particles are usually found within small arteries in the outer wall of the fallopian tube or paratubal tissues and elicit a mild chronic inflammatory response with rare multinucleated giant cells (59). Keratin granulomas secondary to endometrial or ovarian endometrioid adenocarcinoma with squamous differentiation and less commonly squamous cell carcinomas of the cervix or atypical polypoid adenomyomas of the uterus may involve the tubal serosa, fimbria, or lumen (60,61).
Other Types of Granulomatous Salpingitis
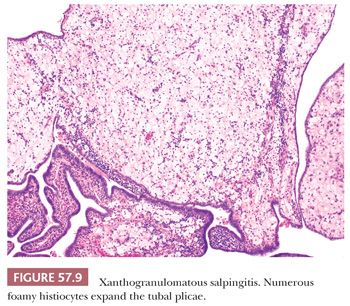
There are rare reports of malacoplakia involving the fallopian tube (62,63). Vasculitides, including giant cell arteritis and polyarteritis nodosa, may also rarely involve the fallopian tube as part of localized or systemic disease (64–68). Necrotizing granulomas of the fallopian tube secondary to diathermy have also been reported (69). Liesegang rings may elicit a chronic or granulomatous inflammatory reaction in the tubal mucosa (70,71). Xanthogranulomatous salpingitis, characterized by mucosal infiltration of numerous foamy histiocytes, may be seen in chronic PID (Fig. 57.9) (72–76). Pseudoxanthomatous salpingitis, characterized by numerous pigmented histiocytes containing lipofuscin, may be encountered in the tubal mucosa secondary to pelvic endometriosis (75,77,78).
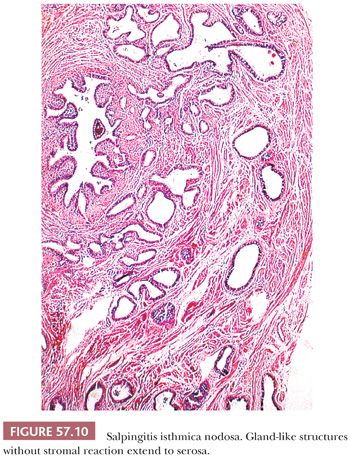
SALPINGITIS ISTHMICA NODOSA
Salpingitis isthmica nodosa consists of diverticula of tubal epithelium involving the myosalpinx of the isthmus. It typically affects young women (mean age, 26 years) and may cause infertility and ectopic tubal pregnancy (79–85). The lesion is often bilateral and appears as yellow-white, firm, rubbery nodularities up to 2.0 cm in diameter involving the isthmus. Dilated diverticula may present as small cysts on cut section. However, lesions may be grossly inconspicuous.
Variably sized and shaped glands lined by a single layer of bland tubal epithelium are present within an often thickened, hyperplastic myosalpinx (Fig. 57.10). Glands may be cystically dilated. Endometrial stroma may occasionally surround glands, and its presence may cause confusion with tubal endometriosis in rare cases. Salpingitis isthmica nodosa is frequently associated with chronic salpingitis which may have a pseudocarcinomatous appearance. However, the regular distribution of glands around the tubal lumen and absence of cytologic atypia or stromal reaction aid in distinguishing this benign lesion from infiltrating carcinoma.
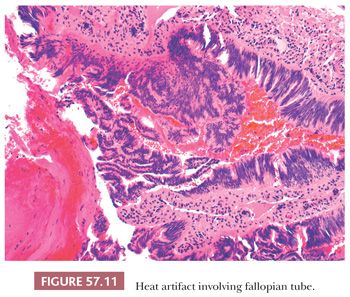
HEAT ARTIFACT
Cautery-induced thermal injury to the fallopian tube demonstrates pseudostratified cells with nuclear elongation and hyperchromasia (Fig. 57.11). These features can mimic adenocarcinoma; however, the streaming appearance of the epithelium is characteristic of thermal artifact (86).
PREGNANCY-RELATED LESIONS
Ectopic Pregnancy
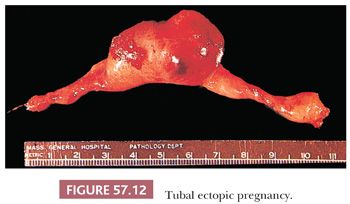
Ectopic pregnancy occurs due to implantation of the developing blastocyst at an extrauterine site. More than 95% of ectopic gestations occur in the fallopian tube, with the vast majority involving the ampulla (70.0%), followed by the isthmus (12.0%), fimbriated end (11.1%), and interstitial portion (2.4%) (87). Rare sites of implantation include the cervix, ovary, and abdomen (87,88). The primary cause of ectopic pregnancy is distortion of the normal tubal anatomy by infection, inflammation, endometriosis, surgery, congenital anomaly, or neoplasm that results in the delayed or aborted passage of the blastocyst into the uterine cavity. Chronic salpingitis and salpingitis isthmica nodosa have been found in 51% to 88% and 14% to 43% of ectopic pregnancies, respectively (82,89). In a long-term, multicenter, prospective cohort study of 10,685 women who underwent tubal ligation, 47 developed ectopic pregnancy with a 10-year cumulative rate of 7.3 per 1000 sterilization procedures (90). Women with in utero diethylstilbestrol exposure have a ninefold increased risk of ectopic pregnancy due to alteration of the tubal anatomy (91). Other risk factors contributing to ectopic pregnancy include prior ectopic pregnancy, previous genital infection, intrauterine device use, infertility, multiple sexual partners, smoking, in vitro fertilization, vaginal douching, and increasing age (92). Abdominal pain, amenorrhea, and vaginal bleeding are classic symptoms of ectopic pregnancy, although over half of women are asymptomatic before tubal rupture (93). The diagnosis is usually made clinically by transvaginal ultrasonography and quantitative serum β-human chorionic gonadotropin (β-hCG) testing.
In the usual tubal pregnancy, distention of the ampullary segment of the tube occurs (94), typically resulting in a sausage-shaped appearance with a thinned or ruptured wall and a dusky red serosa (Fig. 57.12). Rarely, the hemorrhagic mass resulting from the ectopic pregnancy dissects into the broad ligament and may even extend to the contralateral adnexa (95).
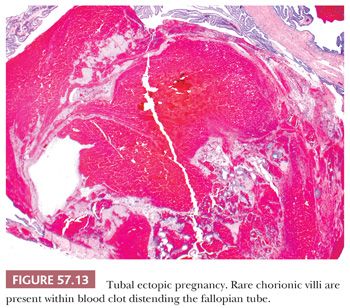
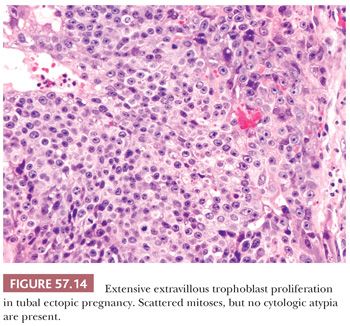
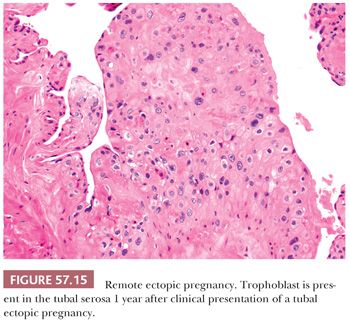
Microscopic examination often reveals viable or necrotic chorionic villi and trophoblast embedded in blood clot which account for the marked luminal distention (Fig. 57.13) (46). Fetal parts may also be identified. Thorough sampling of the intraluminal blood clot for the presence of villi or trophoblast is recommended when ectopic pregnancy is suspected. Tubal placentation is similar to that seen in early first trimester intrauterine pregnancy. Chorionic villi typically consist of polarized trophoblast columns and patchy villus hydrops (96). Trophoblast may invade into the muscularis and subserosa and involve tubal blood vessels with replacement of the vessel wall by fibrinoid material. In late ectopic pregnancies, chorionic villi may transmurally extend into the serosa. The extravillous trophoblast proliferation may be exuberant, forming diffuse sheets, and can mimic gestational trophoblastic neoplasia or hydatidiform mole (Fig. 57.14) (96). However, there is little or no cytologic atypia. In contrast to intrauterine gestations, trophoblast in ectopic pregnancies fails to form a placental disk and develop into a chorion laeve and chorion frondosum (97). Trophoblast from an unsuspected ectopic pregnancy may be retained in the fallopian tube, encountered clinically as an adnexal mass or incidentally on microscopic examination (Fig. 57.15). These lesions are usually composed of intermediate trophoblast associated with hyalinization and non-viable chorionic villi (98). The lamina propria shows decidual change in about a third of ectopic pregnancies (94). Evidence of an underlying predisposing disorder, such as chronic salpingitis, salpingitis isthmica nodosa, endometriosis, or a small tumor, may be present.
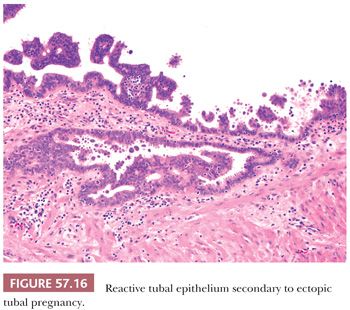
Florid reactive epithelial changes characterized by tufting, nuclear atypia with prominent nucleoli, and occasional hobnail-like cells mimicking a serous borderline tumor may occur; however, minimal to absent mitotic activity is seen (Fig. 57.16). Arias-Stella reaction in the tubal mucosa can be encountered in up to 16% of tubal ectopic pregnancies and in rare intrauterine pregnancies (99,100). Its morphologic features resemble those seen in the endometrium. A rare case of clear cell change involving tubal mucosa associated with ectopic pregnancy has been reported (101). This lesion is similar to its counterpart in the endometrium and does not demonstrate the characteristic nuclear changes of Arias-Stella reaction. Both Arias-Stella reaction and clear cell change should be distinguished from clear cell carcinoma of the fallopian tube which shows malignant features such as cytologic atypia, mitotic activity, and a distinct mass lesion with invasion of the tubal wall.
Hydatidiform Mole
Hydatidiform moles complicating tubal conceptions are exceedingly rare. Both complete and partial moles of the fallopian tube have been reported and display morphologic features identical to those seen in the uterus (96). However, tubal hydatidiform moles are often overdiagnosed due to the presence of an early gestational sac and exuberant extravillous trophoblast proliferation in ectopic pregnancy as described earlier (96,102). The use of strict morphologic criteria, particularly definite, abnormal, circumferential trophoblast proliferation, should be applied when evaluating for molar disease in tubal conceptions. In addition, serial maternal serum β-hCG monitoring and p57 immunohistochemical stains may be helpful. The absence of nuclear p57 expression in villous cytotrophoblast and villous stromal cells is diagnostic of complete mole, although rare, hereditary, biparental complete moles with p57 expression have been reported. The term “invasive mole” should be avoided in the diagnosis of tubal hydatidiform moles and reserved only for cases of persistent gestational trophoblastic disease, that is, chorionic villi invading other tissues, after an ectopic pregnancy (96). Tubal hydatidiform moles have a recurrence rate and risk of development of persistent gestational trophoblastic disease similar to their uterine counterpart (103).
Placental Site Nodule
Extrauterine placental site nodules may be seen in the fallopian tube or paratubal tissues (104–106). Small, circumscribed nodules of intermediate trophoblast associated with prominent hyalinization and absent mitotic activity typically involve the tubal mucosa, but can rarely extend into the wall.
METAPLASIAS AND ECTOPIAS
Endometriosis and Endosalpingiosis
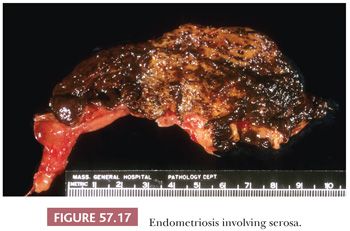
Endometriosis exists as three distinct lesions in the fallopian tube. Most commonly, it refers to serosal or subserosal endometriotic foci associated with endometriosis elsewhere in the pelvis; the myosalpinx is not involved in most of the cases (Fig. 57.17). Occasionally, mucosal involvement is prominent. In some cases of pelvic endometriosis, the mucosal surface appears yellow or brown because of an accumulation of old blood pigment within pseudoxanthoma cells in the lamina propria (78,107).
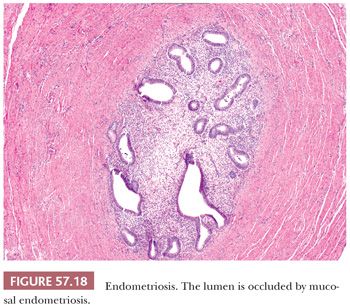
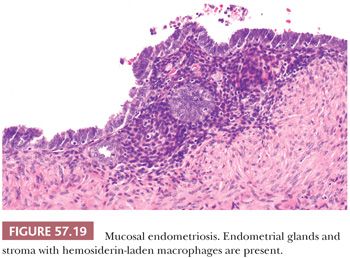
Endometrial tissue may extend directly from the uterine cornu, replacing the mucosa of the interstitial and isthmic portions of the tube in 25% and 10% of women, respectively (Fig. 57.18). This finding is considered a normal morphologic variation, although the ectopic endometrial tissue may give rise to intratubal polyps. The latter have been found in 1% of hysterosalpingographic studies and 11% of hysterectomy and salpingo-oophorectomy specimens (108). The polyps may be associated with ectopic pregnancy and, particularly if they are bilateral, infertility. They are typically unilateral, pink to red lesions, 0.1 to 1.3 cm in diameter that have a smooth surface and a broad-based mucosal attachment. Microscopically, they consist of non-functioning endometrium (109,110). When ectopic endometrial tissue in the interstitial and isthmic portions of the tube causes luminal occlusion, the term “endometrial colonization” has been arbitrarily applied (111–113). The process may represent only an exaggeration of the normal variation described earlier; the involvement may be bilateral. This lesion is said to account for 15% to 20% of cases of infertility, and it may be associated with tubal pregnancy. Cases of endometriosis involving the lining of the fallopian tube (Fig. 57.19) may potentially be misinterpreted as dysplastic simply because of a somewhat darkly staining epithelium with an endometrioid rather than serous character. In the fallopian tube, it is important to look carefully for sometimes subtle foci of endometrial stroma to establish the diagnosis of endometriosis.
The third type of tubal endometriosis has been designated postsalpingectomy endometriosis (114). It occurs at the tip of the proximal tubal stump typically 1 to 4 years after tubal ligation. It is closely related to and may be associated with salpingitis isthmica nodosa. The lesion is analogous to uterine adenomyosis, consisting of endometrial glands and stroma extending from the endosalpinx into the muscularis and frequently to the serosal surface. Hysterosalpingography or India ink injection of a resected specimen may show tubo-peritoneal fistulous tracts (115) through which postligation pregnancies may result. Postsalpingectomy endometriosis has been documented in 20% to 50% of tubes examined after ligation. The frequency of this complication is increased if electrocautery is used, if the proximal stumps are short, and if the postligation interval is long.
Endosalpingiosis involving the fallopian tube consists of ectopic tubal epithelium present on the serosal surface.
Rare Ectopic Tissues
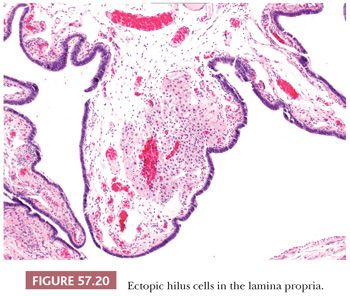
Adrenal cortical rests can be found in the broad ligament in more than 20% of women. Grossly, they appear as small yellow nodules in the infundibulopelvic ligament beneath the peritoneum and may be obscured by fat. Microscopically, all three layers of adrenal cortical tissue are present as seen in the adrenal gland; however, medullary tissue is absent. Adrenal cortical rests may rarely undergo hyperplasia in the setting of excess adrenocorticotropic hormone or give rise to cortical adenomas (116,117). Ectopic hilus cells may be seen in the fallopian tube, paratubal soft tissues, and broad ligament in approximately 0.5% of salpingectomy specimens (118,119). They usually form a discrete nodule in the fimbria, but can appear infiltrative with a corded growth pattern (Fig. 57.20). The presence of cytoplasmic Reinke crystals may be helpful in distinguishing hilus cells from adrenal rests. Rare cases of ectopic pancreas, cartilage, and sex cord inclusions in the fallopian tube have also been reported (120–122).
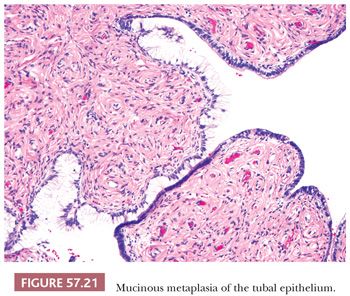
Mucinous Metaplasia
The tubal epithelium can rarely undergo mucinous metaplasia characterized by endocervical-like cells (Fig. 57.21). It may be associated with Peutz-Jeghers syndrome and mucinous tumors of the female genital (ovary, cervix) and gastrointestinal tracts (appendix) (123). However, approximately 50% of mucinous metaplasias of the fallopian tube occur incidentally in the absence of gynecologic or appendiceal neoplasia and are often accompanied by chronic inflammation and/or other metaplastic changes (124). Distinction between mucinous metaplasia and the occasional mucosal spread of a well-differentiated mucinous tumor from the ovary, cervix, or appendix can be difficult, although the clinical and pathologic findings of the latter are usually diagnostic (125).
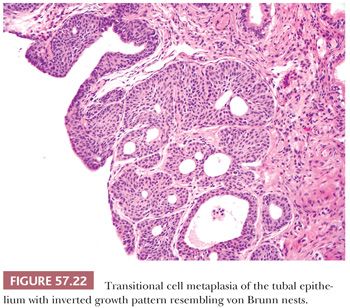
Transitional Cell Metaplasia
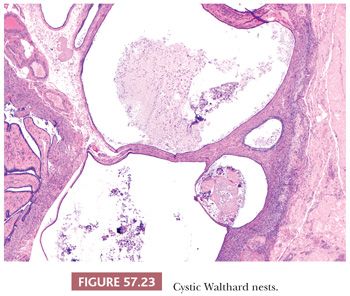
Transitional cell metaplasia of the tubal epithelium is a common incidental finding (126,127) (Fig. 57.22). Walthard nests arising from transitional cell metaplasia often form 1 to 2 mm, yellow to white nodules or cysts involving the tubal serosa, mesosalpinx, and mesovarium, particularly at the columnar-mesothelial junction. Microscopically, they can be solid, plaque-like, or cystic with flocculent eosinophilic or less commonly, mucinous material and attenuated epithelium. They may occasionally be seen in association with tubo-ovarian adhesions (Fig. 57.23). Walthard nests are positive for p63 and GATA3 (128). Squamous metaplasia of the tubal epithelium is also rarely seen.
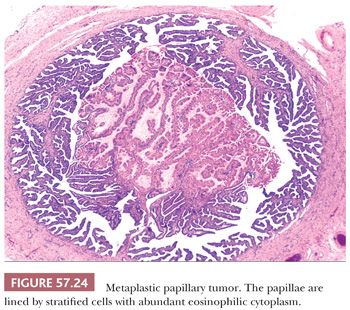
Metaplastic Papillary “Tumor”
Metaplastic papillary “tumor” is rarely found incidentally in fallopian tubes from pregnant and postpartum women, as well as occasionally non-pregnant women (129–132). Intraluminal papillae lined by cuboidal to columnar cells with abundant, eosinophilic cytoplasm which may be associated with intracellular and extracellular mucin are typically seen (Fig. 57.24). Nuclei are bland or at most have only mild atypia with limited pseudostratification. Mitotic figures are rare or absent. It is unclear whether this lesion represents a metaplastic or neoplastic process linked with pregnancy. Short tandem repeat analysis of a single case did not show genetic alterations typically found in ovarian carcinogenesis (133). All cases reported in the literature had a benign clinical course (132).
HYPERPLASIA
Usual Type
Varying degrees of epithelial hyperplasia are frequently seen in the fallopian tube. It can be found in 42% of prophylactic salpingo-oophorectomies in BRCA mutation carriers and up to 83% of routine tubal ligations and salpingectomies performed for benign and malignant disease (134,135). Hyperplasias often have no identifiable cause, but may be associated with various conditions, including inflammation, excess estrogen production, ectopic pregnancy, and neoplasia (136–138). Hyperplasia is often bilateral. The tubal epithelium may show nuclear crowding and stratification, loss of nuclear polarity, tufting, and varying degrees of cytologic atypia (134,135,139). Most cases demonstrate only mild atypia (135), and in such settings, one should not misinterpret the presence of small papillary tufts and nuclear stratification as premalignant change. Mitotic figures are few in number, but often absent. Cells maintain cilia and nuclear-to-cytoplasmic ratio.
In addition to epithelial hyperplasia, mesothelial hyperplasia as well as marked reactive changes can be seen in salpingitis, including those due to both mycobacterial and bacterial causes, and may simulate in situ or invasive adenocarcinoma (140,141). The tubal epithelium can exhibit papillary and gland formations, including cribriform architecture, lined by cells with mild to moderate nuclear pleomorphism, hyperchromatism, and mitotic activity (Fig. 57.25). Hyperplastic epithelial changes may involve the mucosa and extend deep into the muscularis. Epithelial proliferation may be accompanied by florid mesothelial hyperplasia involving inflamed and scarred subserosal tissue, features which may be misinterpreted as transmurally invasive carcinoma (Fig. 57.26). The occasional presence of tubal epithelial clusters within small cysts of mesothelial derivation may be misconstrued as lymphovascular invasion.
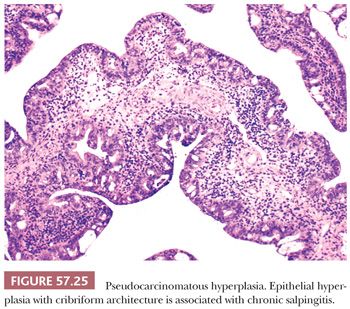
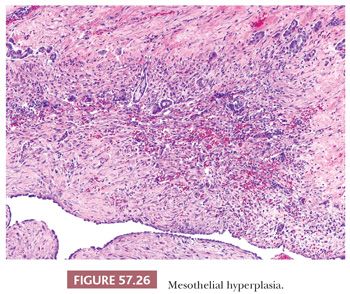
Gross and microscopic features may be helpful in distinguishing between carcinoma and pseudocarcinomatous hyperplasia. Tubal carcinomas often form a mass, whereas pseudocarcinomatous hyperplasia is often an incidental microscopic finding. Carcinomas exhibit brisk mitotic activity and severe cytologic atypia and are usually not associated with extensive inflammation, although there are rare exceptions. When pseudocarcinomatous hyperplasia is accompanied by mesothelial hyperplasia, the mesothelial cells are typically cuboidal and demonstrate only mild nuclear atypia.
Papillary Tubal Hyperplasia and Secretory Cell Outgrowth
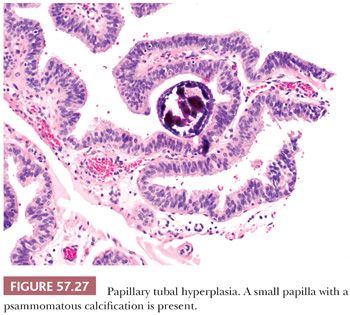
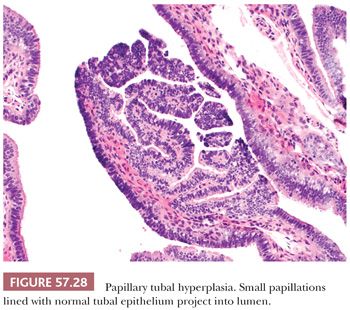
Although tubal epithelial hyperplasia is generally regarded as a frequent incidental finding with no significant clinical significance, its potential association with serous borderline tumors of the ovary has been suggested and is a matter of ongoing debate. Robey and Silva (142) identified tubal epithelial hyperplasia in approximately 70% of patients with ovarian serous borderline tumors, whereas Yanai-Inbar et al. (143) demonstrated the presence of mucosal epithelial proliferation in both normal fallopian tubes and a wide variety of non-neoplastic and neoplastic gynecologic lesions. In a small study of 22 ovarian serous borderline tumors with implants involving the fallopian tube, Kurman et al. (144), however, have recently found that approximately 90% of such tumors are associated with a form of tubal epithelial hyperplasia consisting of intraluminal papillary tufting and detached clusters of bland tubal epithelium with or without psammoma bodies, which they have designated papillary tubal hyperplasia (Fig. 57.27) (144). These morphologic features overlap with those of papillary secretory cell outgrowths (SCOUTs), which also appear more common in the fallopian tubes of patients with serous borderline tumors (Fig. 57.28) (145). However, the morphologic appearance of papillary tubal hyperplasia and papillary SCOUTs is similar to tubal mucosal involvement by serous borderline tumor, and there is no published data regarding the distinction between these two scenarios.
PROLAPSE AND INTUSSUSCEPTION
Tubal prolapse into the vaginal vault is an uncommon but known complication of vaginal or abdominal hysterectomy, often secondary to incomplete peritoneal closure or postoperative infection or hematoma (146–149). A polypoid mass resembling granulation tissue is usually present at the vaginal apex, with fimbria grossly identifiable in some cases. Tubal epithelium in a background of inflammation is often observed and can mimic adenocarcinoma when there is severe cytologic atypia, papillary architecture, or pseudoglandular formation (150). However, the presence of plicae and ciliated cells of benign tubal epithelium are clues to the correct diagnosis. The stroma in the prolapsed fallopian tube may occasionally exhibit an exuberant angiomyofibroblastoma-like response (151) or mimic aggressive angiomyxoma (152).
Intussusception of the fallopian tube is rare and may occur after suction curettage, resulting in infarction (153).
NEOPLASMS OF THE FALLOPIAN TUBE
BENIGN NEOPLASMS
Adenomatoid Tumor
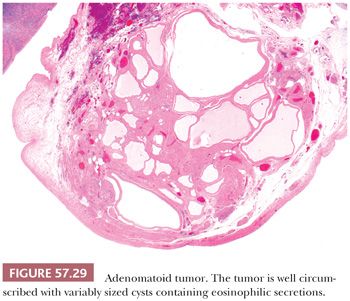
Adenomatoid tumor is the most frequent benign neoplasm of the fallopian tube, often found incidentally in peri- or postmenopausal women. Grossly, they are typically less than 2.0 cm in diameter, circumscribed, firm, white, gray, or yellow mural nodules that may project from the tubal serosa or compress the lumen. Tumors are rarely bilateral. Adenomatoid tumors presumably arise from mesothelium, with rare cases demonstrating continuity with the overlying serosa. Histologically, tubal adenomatoid tumors resemble their uterine counterpart but lack the marked smooth muscle proliferation typical of the latter entity. Microscopic patterns include small, slitlike to round and cystically dilated glandlike spaces, cords, clusters, and sheets of neoplastic cells present within the muscular wall of the fallopian tube (Fig. 57.29). Cells range from flattened mimicking endothelium to cuboidal with abundant eosinophilic cytoplasm containing inconspicuous vacuoles (Fig. 57.30). Nuclei are round and bland, and mitotic figures are rare or absent. Glandlike spaces may be empty or contain slightly basophilic secretions. The stroma may be edematous, fibrous, or hyalinized and can contain smooth muscle. Chronic inflammation may be present and form prominent lymphoid follicles (154,155).
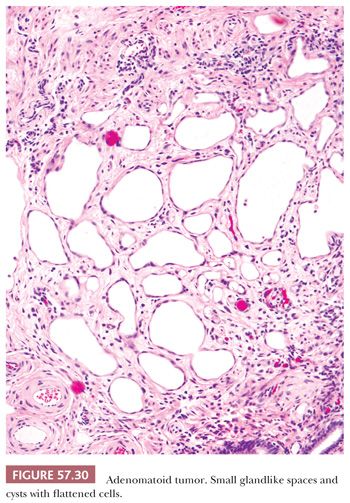
Histochemical stains often reveal presence of hyaluronic acid mucin within vacuoles and glandlike spaces (156). Adenomatoid tumors express pankeratin as well as mesothelial markers, such as calretinin, D2-40, and WT-1 (154,156–159). They may rarely be positive for CK5/6 and caldesmon (154). PAX8, CD34, estrogen receptor (ER), progesterone receptor (PR), and epithelial membrane antigen (EMA) expression are usually absent (159,160). Abundant microvilli characteristic of mesothelial differentiation can be seen on electron microscopy (156,161).
The differential diagnosis includes other benign lesions, such as lymphangioma and leiomyoma. Cytokeratin expression and absence of endothelial markers distinguish adenomatoid tumors from vascular neoplasms. Although adenomatoid tumor may mimic leiomyoma macroscopically and contain smooth muscle, the presence of characteristic glandlike spaces is lacking in smooth muscle neoplasms. Adenomatoid tumors may also be confused with malignancy, particularly malignant mesothelioma and adenocarcinoma, especially of signet-ring cell type. They may also rarely undergo massive infarction in which pseudoinfiltration at the periphery, necrosis, and reactive atypia may obscure their typical morphologic appearance and raise the possibility of a malignant process (162). However, gross tumor circumscription, bland cytologic features, and lack of mitotic activity facilitate the diagnosis of adenomatoid tumor. In the setting of infarction, the presence of viable tumor or ghost outlines of typical patterns allows distinction from malignancy.
Epithelial Tumors
The endometrioid polyp is the most common benign epithelial tumor of the fallopian tube and occurs in 2% to 3% of women who undergo hysterosalpingography (163). Tubal polyps typically involve the interstitial portion of the fallopian tube and are histologically identical to endometrial polyps. They are often small in size, but may cause tubal occlusion, resulting in infertility or ectopic pregnancy (110,163,164). Tubal polyps presumably arise from mucosal endometrial tissue and may be associated with endometriosis (163).
Papillomas of the tube are rare and consist of a complex, papillary, and adenomatous proliferation of tubal epithelium (165,166). Thin fibrovascular cores are lined by bland ciliated and secretory cells with no mitotic activity.
Rare adenomas and cystadenomas of mucinous and clear cell types have been reported (123,167).
Borderline Epithelial Tumors
Primary borderline tumors of the fallopian tube have been reported, but are rare. Most are of the serous type (168–172). Neoplasms considered examples of primary tubal mucinous borderline tumor have been described, many of which were associated with pseudomyxoma peritonei (123,173,174). However, tubal spread from a mucinous cystic tumor of the appendix was not excluded in some of these cases in which the appendix was not examined histologically. A single endometrioid borderline tumor involving the fimbria has been reported (171).
Mixed Epithelial-Mesenchymal and Mesenchymal Tumors
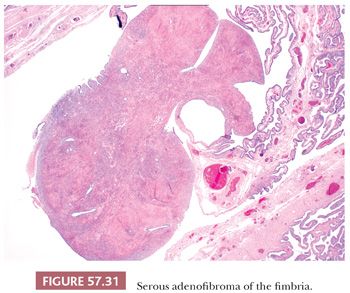
Adenofibromas often involve the fimbria and are morphologically similar to their ovarian counterpart (Fig. 57.31). They are usually less than 1 cm in size, incidental, and present in 30% of salpingectomies (175). The epithelial component is commonly serous, although occasionally endometrioid (171). The stroma may be hypercellular, hyalinized, or fibrotic and, like ovarian adenofibromas, expresses inhibin and CD10 (175). Some can be associated with an ovarian adenofibroma (175). Rare cystadenofibromas and adenomyomas of the fallopian tube have been reported (176,177).
Leiomyoma is the most common mesenchymal neoplasm of the fallopian tube (178). Tumors are usually small in size and can be submucosal, intramural, or subserosal. Similar to their uterine counterpart, tubal leiomyomas may undergo degenerative changes. Other mesenchymal tumors occasionally found in the fallopian tube include angiomyolipoma (179), lipoma (180), extraskeletal chondroma (181–183), angiomyofibroblastoma (184), and hemangioma (185–187).
Neuroectodermal Tumors
Rare neuroectodermal tumors of the fallopian tube, including schwannoma and ganglioneuroma, have been described and are morphologically similar to their counterparts at other sites (188–190).
Germ Cell Tumors
Germ cell tumors of the fallopian tube are uncommon, and most examples have been teratomas. Tubal teratomas are usually intraluminal and attached by a pedicle to the mucosa, but can rarely be intramural or involve the serosa. They range in size from 0.7 to 31 cm in diameter (191). The majority of tubal teratomas are mature and cystic, although they can occasionally be solid (191–193). Rare examples composed entirely of mature thyroid tissue have been described (194). Carcinoid arising in a mature tubal teratoma as well as a mixed immature teratoma and yolk sac tumor of the fallopian tube have been described (195,196).
MALIGNANT NEOPLASMS OF THE FALLOPIAN TUBE
Carcinoma
Primary carcinoma of the fallopian tube has traditionally been considered rare and accounts for only 0.7% to 1.5% of all gynecologic malignancies (197,198), with an incidence ranging from 0.3 to 0.5 per 100,000 women (199–203). These figures, however, are likely an underestimation of its true frequency due to possible misclassification of carcinomas of uncertain origin involving both ovary and fallopian tube as ovarian primaries as well as recent evidence suggesting that some “ovarian” serous carcinomas may be of tubal origin.
Patients with primary tubal carcinoma have a wide age range (25 to 95 years), but the vast majority are postmenopausal with a mean age of 61 years (204–206). The most common symptom at clinical presentation is abnormal vaginal bleeding or discharge, followed by abdominal pain, a palpable pelvic and/or abdominal mass, and suspected ascites (204–206). Serum CA-125 is elevated in most patients (204,206), although it may be normal in those with tubal intraepithelial carcinoma (TIC). The classic symptom of hydrops tubae profluens—intermittent, colicky abdominal pain relieved by profuse, watery vaginal discharge—considered pathognomonic of tubal carcinoma is infrequent (204–206). A subset of patients may present with adenocarcinoma or atypical cells found on endometrial or endocervical sampling or Pap smear of the lower genital tract. Tubal carcinoma is discovered incidentally in a small subset of patients who undergo surgery for other non-neoplastic gynecologic disorders (206). A small percentage of patients may present with an associated paraneoplastic syndrome (207–211). In rare cases, the presenting clinical symptom of an occult tubal carcinoma is distant metastasis, such as lymph node involvement or malignant pleural effusion (204,212,213). Occasional tubal carcinomas that are associated with marked inflammation may present as acute PID (214,215). A subset of patients has a history of another malignancy, particularly breast cancer (204). Due to the rarity of tubal carcinomas and a clinical presentation that is often non-specific and overlaps with that of ovarian cancer, a correct preoperative diagnosis is exceptionally rare.
Tubal carcinoma is now encountered with increased frequency in patients with deleterious germline mutations involving BRCA1 or BRCA2 genes. Carriers of these mutations also have higher risk of developing breast, ovarian, and peritoneal carcinomas. The frequency of hereditary tubal carcinoma is currently unknown. However, germline mutations have been detected in 15% to 43% of patients with tubal carcinoma (216–219). The lifetime risk of developing tubal carcinoma is 0.6 in BRCA mutation carriers (218) and 0.2 in the general population (199). Genetic counseling and risk-reducing bilateral salpingo-oophorectomy (RRSO) after completion of childbearing have thus been recommended for women at highest risk for ovarian and tubal carcinomas. RRSO has been considered to significantly reduce the risk of gynecologic cancers. The relative risk of ovarian/tubal/peritoneal carcinoma after RRSO is reportedly 0.04 (220). In another study, the relative risk of breast/ovarian/tubal/peritoneal carcinoma after RRSO is 0.25 (221).
Thorough pathologic examination of ovaries and fallopian tubes in their entirety has been recommended to enhance detection of microscopic lesions in RRSO specimens from patients with BRCA mutations or a strong family history. Sectioning and extensively examining the fimbria (SEE-FIM protocol), the area in which most early tubal carcinomas arise, maximizes the total surface area of tubal epithelium available for histologic examination (222). This has become the standard of care in processing RRSO specimens and includes the following steps: immediate fixation in formalin upon receipt of the specimen to minimize loss of epithelium due to manipulation, transection of the fimbriated end and sectioning longitudinally at 2 mm intervals, transverse sectioning of the remainder of the fallopian tube and entire ovary at 2 mm intervals, and submission of the entire fallopian tube and ovary for microscopic examination (222). Intraoperative dissection or frozen section of RRSO specimens that are grossly normal should be avoided (223). Although additional level sections of the fimbriated end may slightly improve detection of occult tubal carcinoma (224,225), further serial sectioning is recommended only in anecdotal cases if an atypical focus of tubal epithelium is encountered on the initial hematoxylin and eosin (H&E) slide. The rate of detecting invasive or intraepithelial carcinoma of the fallopian tube in RRSO specimens from BRCA mutation carriers ranges from 2% to 10% (134,224,226–232).
The vast majority of tubal carcinomas are unilateral, with bilaterality observed in only 2% to 13% of cases (199,204–206,233). Tumors range in size from 0.1 to 10.0 cm with an average diameter of 4.3 cm (204,205,224). In many examples, there is fusiform swelling of the fallopian tube that may mimic hydrosalpinx, hematosalpinx, or pyosalpinx. However, in other cases, the tube is not appreciably enlarged. Small fimbrial tumors may be seen occasionally and can often be initially overlooked upon gross examination. Staging of fallopian tube carcinoma is according to both tumor-node-metastasis and the International Federation of Gynecology and Obstetrics (FIGO) classifications, and modifications of the latter have been proposed to better reflect these changes in our understanding of the histogenesis of high-grade serous carcinomas (13). Carcinomas of ovary, fallopian tube, and peritoneum are now staged using identical criteria (Table 54.3). In some advanced-stage high-grade serous carcinomas, it is impossible to assign a primary site with certainty (i.e., primary ovarian, tubal, or peritoneal carcinoma), but with the updated staging criteria, this will not impact on stage assignment, nor is treatment changed based on primary site.
Carcinomas of various surface epithelial types encountered in the ovary have been reported in the fallopian tube, but the frequency of these neoplasms in the tube differs from those in the ovary. Over half of tubal carcinomas are serous (204–206). Endometrioid carcinoma accounts for the second most common subtype (204–206). Other histologic types, including clear cell, mucinous, and undifferentiated carcinomas, are rare (204–206). Tubal carcinomas share similar morphologic features with their ovarian counterparts and are described in detail in the following discussion.
Serous Carcinoma. Serous tubal intraepithelial carcinoma (STIC) is the earliest form of serous carcinoma found in the fallopian tube and may be involved in the pathogenesis of high-grade serous carcinomas of the ovary and peritoneum. Lesions regarded as severe dysplasia or carcinoma in situ in the literature fall into this category. However, the use of such terminology is currently discouraged as both imply that there is no potential for metastasis.
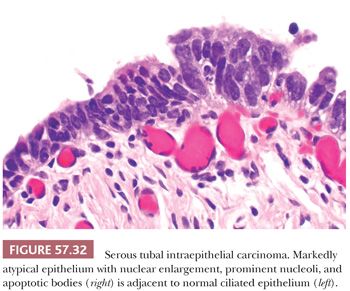
Histologically, STICs are characterized by markedly atypical, non-ciliated tubal epithelium without invasion of the underlying lamina propria. Cytologic abnormalities include nuclear enlargement and pleomorphism, high nuclear-to-cytoplasmic ratio, hyperchromatism or uneven chromatin pattern, prominent nucleoli, and nuclear stratification (Fig. 57.32). The threshold for diagnosing STIC should be high, as normal tubal epithelium may show some degree of atypia or tufting (139). The lesional epithelium is often flat, but may show uneven luminal contours or incipient papillae or detached cells (Fig. 57.33). The atypical epithelium can also invaginate into the lamina propria, causing confusion with invasion (Fig. 57.34). Mitotic figures are usually present, and apoptotic bodies may be seen. The lesion may be a single focus or multifocal. In some cases, STIC may be adjacent to invasive carcinoma. Only few STICs are recognizable at low magnification, appearing darker than the adjacent normal tubal epithelium; thus, screening of RRSO specimens at intermediate magnification is recommended. Before diagnosing STIC, it is prudent to submit all remaining fallopian tube tissue to exclude a microscopic focus of invasive carcinoma. Any lesion that does not meet criteria of the classic cytologic aberrations as described earlier should be conveyed descriptively in the pathology report.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


