Chapter 40 Estrogens and Progestins
| Abbreviations | |
|---|---|
| CBG | Corticosteroid-binding globulin |
| ER | Estrogen receptor |
| ERT | Estrogen replacement therapy |
| FSH | Follicle-stimulating hormone |
| GI | Gastrointestinal |
| GnRH | Gonadotropin-releasing hormone |
| HDL | High-density lipoprotein |
| HRE | Hormone responsive element |
| IM | Intramuscular |
| IUD | Intrauterine devices |
| LDL | Low-density lipoprotein |
| LH | Luteinizing hormone |
| PGR | Progesterone receptor |
| SERM | Selective estrogen receptor modulator |
| SHBG | Sex hormone-binding globulin |
Therapeutic Overview
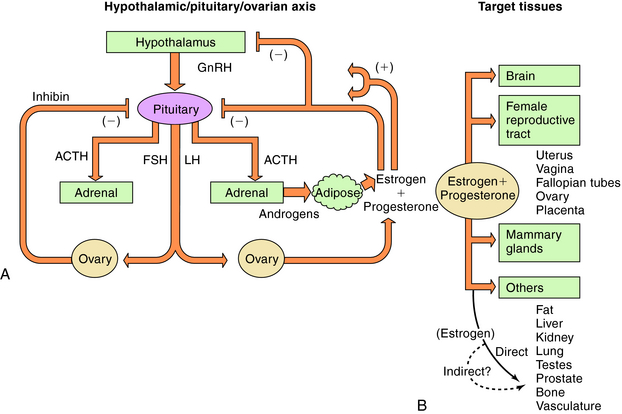
There are three endogenous nineteen-carbon steroids in humans that have estrogenic activity. The principal ovarian estrogens are 17β-estradiol, which is the primary circulating form, and its metabolite, estrone, which the primary postmenopausal estrogen. During pregnancy the placenta synthesizes estriol. Estrogens coordinate systemic responses during the ovulatory cycle, including regulation of the reproductive tract, pituitary, breasts, and other tissues. Also, some forms of cancer are estrogen-dependent for growth. The hypothalamic-pituitary-ovarian axis and target organs for the actions of estrogens are shown in Figure 40-1. Estrogens are also responsible for mediating development of secondary sex characteristics when females enter puberty, including progressive maturation of the fallopian tubes, uterus, vagina, and external genitalia. Upon estrogenic stimulation, more fat is deposited in the breast, buttocks, and thighs, leading to the normal adult female habitus. The following are characteristics promoted by estrogens.
The important endogenous progestin is progesterone, although 17α-, 20α-, and 20β-hydroxyprogesterones have weak progestational activities. Estrogen priming is necessary for progesterone receptor (PGR) expression in almost all progesterone-responsive tissues, including the uterus. Progesterone concentrations rise rapidly in the luteal phase of the menstrual cycle, resulting in a modulation of the action of estrogen on the uterus. Progesterone antagonizes estrogen-induced proliferation in the uterus and initiates secretory changes in preparation for embryo implantation. In the absence of pregnancy, plasma progesterone concentrations decrease, resulting in sloughing of the endometrial lining. Progesterone is responsible for causing the increased basal body temperature observed in the luteal phase. Progesterone is important in mammary glandular development and, unlike the uterus, probably stimulates breast cell proliferation. During pregnancy, progesterone can promote maintenance of pregnancy, inhibit uterine contraction, alter carbohydrate metabolism, decrease HDL, increase LDL, and increase Na+ and water elimination by competitive antagonism of aldosterone interaction with mineralocorticoid receptors. A variety of menstrual cycle disorders are treated with progestins, estrogens, or both.
| Therapeutic Overview |
|---|
| Fertility Control |
| Combination contraception (estrogens plus progestins) |
| Progestin-only contraception |
| Emergency contraception (estrogens plus progestins, progestins) |
| Contragestation (antiprogestin) |
| Infertility Treatment |
| Ovulation induction (SERMs, GnRH analogs, gonadotropins) |
| Replacement Therapy |
| Acute symptoms of menopause (estrogens plus progestins, estrogens) |
| Prevention of osteoporosis (SERMs, estrogens) |
| Ovarian failure (estrogens plus progestins) |
| Dysfunctional uterine bleeding (progestins, estrogens plus progestins) |
| Luteal phase dysfunction (progestins) |
| Cancer Chemotherapy |
| Breast cancer adjuvant treatment (SERMs, aromatase inhibitors, steroidogenesis inhibitors) |
| Advanced breast cancer (aromatase inhibitors, SERMs) |
| Advanced endometrial cancer (progestins) |
| Advanced prostate cancer (estrogens) |
| Breast cancer prevention (SERMs) |
| Others |
| Endometriosis (estrogens plus progestins, progestins, progesterone analog, progestin plus GnRH analog) |
| Dysfunctional uterine bleeding (progestins, estrogens plus progestins) |
| Luteal phase dysfunction (progestins) |
Mechanisms of Action
Biosynthesis of Estrogens and Progestins
Estrogens and progestins are produced by steroidogenesis in various tissues (see Fig. 38-1). The ovary is the predominant source of these steroids in nonpregnant, premenopausal women. A significant amount of estrogenic activity is also produced by skeletal muscle, liver, and adipose tissue through the conversion of circulating androgens to estrone. Certain brain areas in males and females may also produce estrogens through the action of aromatase on circulating androgens. Small amounts of estradiol can be produced in the male testes.
During the menstrual cycle, the pituitary gonadotropins FSH and LH regulate the synthesis and release of estrogen and progesterone from the ovary. The pulsatile release of hypothalamic gonadotropin-releasing hormone (GnRH), in turn, regulates FSH and LH synthesis and release. GnRH concentrations are regulated through negative and positive feedback by the steroid hormones. Estrogens and progestins also act directly on the pituitary gonadotrophs to decrease FSH and LH concentrations. In addition, an ovarian protein, inhibin, negatively affects FSH synthesis. The pathways for the integrated control of hormone regulation are shown in Figure 40-1, A.
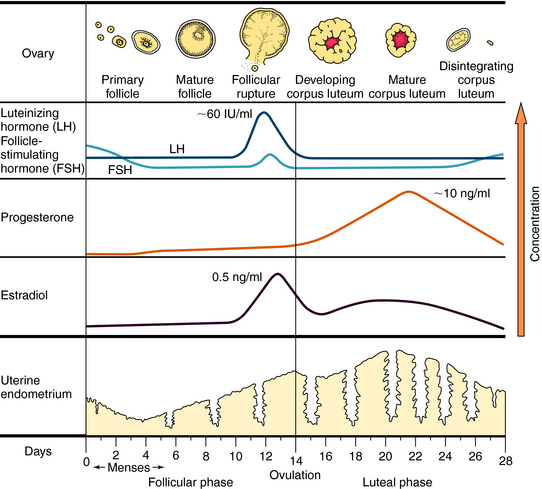
The ovulatory-menstrual cycle normally spans 25 to 35 days. The steps in the ovarian and endometrial cycles are shown in Figure 40-2. The ovarian cycle is divided into the follicular (preovulatory) phase, when ova maturation and estrogen release occurs, ovulation, when follicular rupture leads to ova release, and the postovulatory phase, when the corpus luteum maximally releases progesterone and stimulates growth of the endometrial lining. The follicle is the basic reproductive unit of the ovary and consists of an oocyte surrounded by granulosa cells. At the onset of a menstrual cycle, FSH accelerates maturation of several follicles. Through interactions with its receptor, FSH increases aromatase activity, which stimulates conversion of androgens to estradiol. By days 8 to 10, FSH decreases, and the dominant follicle becomes more sensitive to circulating gonadotropin because of an increased number of FSH receptors. In the late follicular phase, estradiol levels increase rapidly and initiate a mid-cycle LH surge (16 to 24 hours before ovulation). Increased LH levels promote follicular production of progesterone, prostaglandin F2α, and proteolytic enzymes, and ultimately, follicular rupture and ovulation occur. After ovulation, the granulosa and theca cells become the corpus luteum, which produces and releases progesterone throughout the first half of the luteal phase (10 to 20 ng/mL). The suppression of FSH and LH release promotes the decline of progesterone and estrogen, luteolysis, initiation of menses, and ultimately a new cycle.
Compounds with estrogenic activity can be classified as either steroidal or nonsteroidal. Steroidal estrogens can be subdivided into natural and synthetic forms. The structures of progesterone, the three endogenous human estrogens (β-estradiol, estrone, and estriol), and their biosynthetic pathways are shown in Figures 38-1 and 38-2. Estradiol is the most potent of the three estrogens. Synthetic hormones that are used therapeutically generally have a heterocyclic structure resembling endogenous steroids.
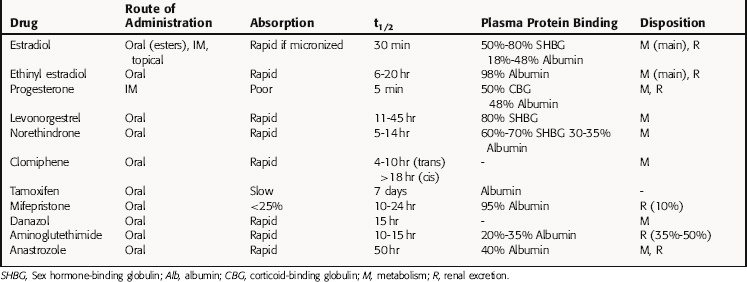
The synthetic estrogens, ethinyl estradiol and mestranol, are used predominantly in combination oral contraceptives. These compounds have an ethinyl group at C17, which retards hepatic inactivation. Mestranol requires activation by hepatic conversion to ethinyl estradiol. More recently, attention has focused on the nonsteroidal synthetic compounds, selective estrogen receptor modulators (SERMs), that interact with estrogen receptors (ER). The first available SERM, tamoxifen, is a triphenylethylene derivative that acts as an estrogen antagonist in breast; clinical applications include treatment, and recently prevention, of breast cancer (see Chapter 55). A clinically important benefit of tamoxifen is its agonist activity in bone, which antagonizes osteopenia. A concern with tamoxifen is the significantly increased risk of endometrial cancer and venous thrombosis related to its estrogenic activity. Raloxifene is a SERM developed to delay osteoporosis in postmenopausal women who are not candidates for estrogen treatment. It has agonist activity in bone but displays little estrogen-like activity in breast or uterus. Clomiphene citrate is a racemic mixture of two stereoisomers that has both agonist and antagonist properties and is used to treat infertility by inducing ovulation. Considerable research is directed at identifying new SERMS with tissue-specific agonist and antagonist properties for each therapeutic goal.
Two additional important ligands of the PGR include danazol and mifepristone (formerly called RU486). Danazol is a steroid derivative that has significant agonist activity at both progesterone and androgen receptors and is used in treating endometriosis. Mifepristone is a steroid derivative that binds to both progesterone and glucocorticoid receptors and displays progestin antagonist activity in most target tissues.
The molecular basis for hormone action, including estrogen and progesterone, is reviewed in Chapter 1. Free steroid passively diffuses into any cell but accumulates only in cells expressing the specific cytoplasmic steroid-binding proteins. Both estrogen and progesterone receptors are members of the nuclear receptor superfamily. The two distinct ER subtypes, ERα and ERβ, are products of different genes, and estrogen binds with high affinity to both receptor subtypes. ERα is expressed in the reproductive tract and breast and mediates many of the effects of estrogen on sexual development and reproductive function. ERβ is highly expressed in ovary and brain. All currently available drugs that target estrogen receptors can bind to both receptor dimers, suggesting that selectivity of action is not simply dependent on the presence of receptor subtypes. Because the ligand-binding domains of these two ER subtypes are different, specific ligands for each receptor are likely to be developed. Only one gene encodes the PGR, but two protein isoforms are produced, PGR-A and PGR-B. These proteins display some functional differences in experimental systems, and their ratio shows some variability. However, they have the same ligand-binding domain, and pharmacological effects result from activation of both isoforms.
The classical mechanism of action of nuclear steroid hormones is that the steroid-hormone complex can act as a steroid-activated transcription factor (see Chapter 1). The response of a tissue to a specific ligand is highly regulated at multiple levels: