Endocrine System Disorders
Learning Objectives
After studying this chapter, the student is expected to:
1. Explain the homeostasis of hormones.
2. Name the endocrine glands and the hormones they secrete.
3. Describe the function of each hormone discussed.
4. Differentiate the action of steroid and nonsteroid hormones on the target cell.
5. Explain the relationship between metabolic syndrome and diabetes mellitus.
6. Differentiate Type 1 and Type 2 diabetes mellitus.
7. Compare the causes and development of hypoglycemia and hyperglycemia.
8. Describe the common degenerative effects of diabetes mellitus.
10. Describe the possible effects of a pituitary tumor.
11. Compare the effects of an excess and a deficit of growth hormone in a child and an adult.
12. List the causes and effects of diabetes insipidus and inappropriate ADH syndrome.
13. Describe the causes of goiter.
14. Explain the effects of an excess and a deficit of thyroid hormones.
15. List the possible causes of Cushing’s syndrome.
16. Compare the effects of Cushing’s and Addison’s diseases.
Key Terms
anabolic
catabolism
ectopic
endemic
gluconeogenesis
glucosuria
hyperglycemia
hypoglycemia
iatrogenic
ketoacidosis
ketones
ketonuria
macroangiopathy
microangiopathy
negative feedback
neuropathy
polydipsia
polyphagia
polyuria
tropic
Review of the Endocrine System
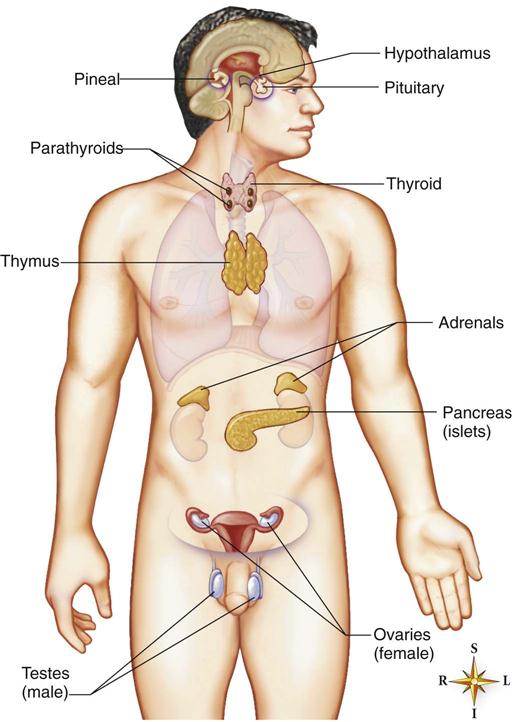
The major endocrine glands are scattered throughout the body and include the hypothalamus, pituitary gland (hypophysis), pineal gland, the two adrenal glands, the thyroid gland, the four parathyroid glands, the endocrine portion of the pancreas, the gonads, and the thymus (Fig. 16-1). Also, local hormones are secreted in the digestive tract, which regulates its secretions and motility. These hormones are discussed in Chapter 17. Endocrine glands secrete hormones directly into the blood, in contrast to exocrine glands that secrete into a duct, such as mucus, serous glands, or pancreatic digestive enzymes.
Hormones are chemical messengers that may be classified by action, source, or chemical structure. For example, several hormones affect blood glucose levels, including insulin, glucagon, epinephrine, cortisol, and growth hormone.
• Releasing and inhibiting hormones
• Antidiuretic hormone (ADH—vasopressin)
• Oxytocin
• FSH—follicle stimulating hormone
• TSH—thyroid stimulating hormone
• ACTH—adrenocorticotropic hormone
• Thymus
• Thyroid
• Epinephrine and norepinephrine
• Pancreas
• Insulin
• Glucagon
• Ovaries
• Estrogen
• Testis
• Classification by chemical structure
• Steroid
• Need a second messenger system to finally activate the formation of mRNA
Following release from an endocrine gland, the hormones circulate to target cells in other glands or tissues. After acting on specific receptors on or in the target cells, the hormones are metabolized or inactivated by the target tissues or the liver and excreted by the kidneys to prevent excessive amounts from accumulating in the body over a period of time. Table 16-1 provides a brief review of major hormones, their sources, and primary effects.
TABLE 16-1
Sources of Major Hormones and Primary Effects
| Hormone | Source | Primary Effects |
| Hypothalamic-releasing hormones | Hypothalamus | Stimuli to anterior pituitary to release specific hormone |
| Hypothalamic-inhibiting hormones | Hypothalamus | Decrease release of specific hormone by anterior pituitary |
| Growth hormone (GH, somatotropin) | Pituitary—anterior lobe (adenohypophysis) | Stimulates protein synthesis |
| Adrenocorticotropic hormone (ACTH) | Adenohypophysis | Stimulates adrenal cortex to secrete primarily cortisol |
| Thyroid-stimulating hormone (TSH) | Adenohypophysis | Stimulates thyroid gland |
| Follicle-stimulating hormone (FSH) | Adenohypophysis | Women: stimulates growth of ovarian follicles and estrogen secretion; men: stimulates sperm production |
| Luteinizing hormone (LH) | Adenohypophysis | Women: stimulates maturation of ovum and ovulation; men: stimulates secretion of testosterone |
| Prolactin (PRL) | Adenohypophysis | Stimulates breast milk production during lactation |
| Antidiuretic hormone (ADH, or vasopressin) | Pituitary- posterior lobe (neurohypophysis) | Increases reabsorption of water in kidney |
| Oxytocin (OT) | Neurohypophysis | Stimulates contraction of uterus after delivery Stimulates ejection of breast milk during lactation |
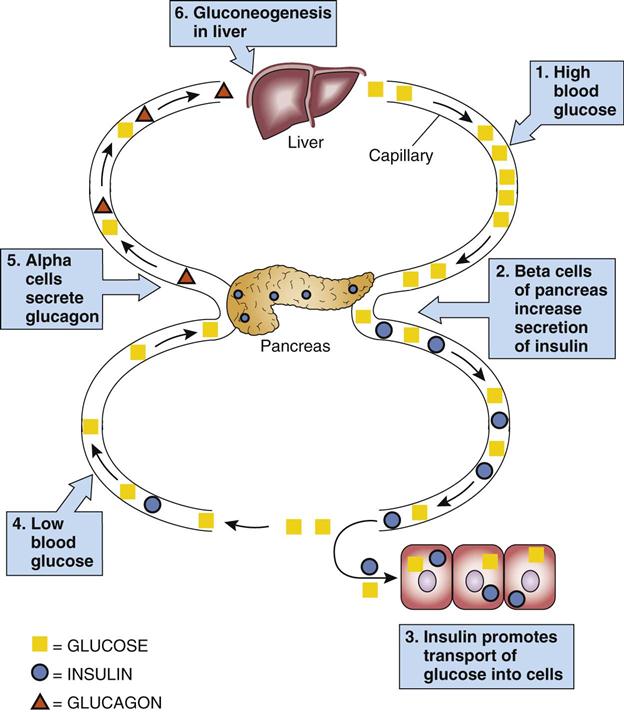
| Insulin | Pancreas—beta cells of islets of Langerhans | Transport of glucose and other substances into cells Lowers blood glucose level |
| Glucagon | Pancreas—alpha cells | Glycogenolysis in liver Increases blood glucose level |
| Parathyroid hormone (PTH) | Parathyroid gland | Increases blood calcium level by stimulating bone demineralization and increasing absorption of Ca++ in the digestive tract and kidneys |
| Calcitonin | Thyroid gland | Decreases release of calcium from the bone to lower blood calcium level |
| Thyroxine (T4) and triiodothyronine (T3) | Thyroid gland | Increases metabolic rate in all cells |
| Aldosterone | Adrenal cortex | Increases sodium and water reabsorption in the kidney |
| Cortisol | Adrenal cortex | Anti-inflammatory and decreases immune response Catabolic effect on tissues; stress response |
| Norepinephrine | Adrenal medulla | General vasoconstriction |
| Epinephrine | Adrenal medulla | Stress response Visceral and cutaneous vasoconstriction Vasodilation in skeletal muscle Increases rate and force of heart contraction Bronchodilation |
The release of hormones from glands is most frequently controlled by a negative feedback mechanism (Fig. 16-2). For example, as levels of glucose increase, the secretion of insulin increases. When glucose levels decrease, insulin secretion decreases.
The endocrine and nervous systems work together to regulate metabolic activities. The hypothalamus and pituitary gland comprise a complex control system for some hormones. The hypothalamus initially secretes releasing or inhibiting hormones; for example, thyrotropin-releasing factor acts on the pituitary gland to secrete thyroid-stimulating hormone (TSH). When determining the cause of a hormonal deficit or excess, it is necessary to check pituitary hormone levels as well as those of the target gland. For example, a deficit of thyroxine could result from a pituitary problem (decreased secretion of TSH) or a problem in the thyroid gland. In the latter case, blood levels of TSH would be high, whereas thyroxine levels would be low (see Fig. 16-13 for the thyroid hormone feedback system).
In some cases, secretion is controlled by more than one mechanism (e.g., aldosterone is regulated by renin secretion and serum levels of Na+ and K+).
To assist in maintaining a well-controlled blood level of a substance such as calcium, a balance of several hormones, such as parathyroid hormone and calcitonin, is required. These are antagonistic hormones and have opposing actions on serum calcium. The blood level of glucose is critical to nervous system function and fluid balance; therefore, as mentioned, it is controlled by a number of hormones.
Another variable affecting hormone levels in the body is the rate or timing of secretion. Some hormones, such as thyroid hormone, are maintained at fairly constant levels, whereas others are released in large amounts intermittently as the demand occurs. Some hormones, such as estrogen, follow a cyclic pattern in women. Adrenocorticotropic hormone (ACTH) and cortisol are secreted in a diurnal pattern, the highest levels occurring in the morning and the lowest levels at night. If an individual’s sleep pattern changes, the hormonal secretion changes with it. However, any acute stress leads to the sympathetic nervous system overriding this pattern, resulting in a great outflow of ACTH and cortisol.
Endocrine Disorders
There are essentially two categories of endocrine problems: an excessive amount of hormone and a deficit of hormone. The manifestations of hormonal disorders reflect the actions of the hormone. Frequently endocrine disorders cause distinctive changes in the individual’s physical appearance, which may be helpful in diagnosis.
The most common cause of endocrine disorders is the development of a benign tumor, or adenoma. Adenomas may be secretory, causing excess hormone, or they may have a destructive effect on the gland, causing a hormonal deficit. In the case of an adenoma in the pituitary gland, located in the bony sella turcica on the inferior surface of the brain, this will cause increased intracranial pressure and neurologic effects.
In some cases, the target cells are resistant or insensitive to the hormone, thus creating the effect of a hormone deficit. This lack of response may result from a genetic defect, an autoimmune response, or excessive demand on the target cells. An example of this receptor insensitivity to insulin may be seen in Type 2 diabetes mellitus.
Other causes of hormonal imbalances include congenital defects in the glands, hyperplasia or infection in the glands, abnormal immune reactions, and vascular problems.
Not all hormones are covered in this chapter. However, if the normal effects of a hormone are known, it is possible to predict the effects of an excess or a deficit. Diagnostic tests and treatment follow similar patterns.
Diagnostic Tests
Levels of tropic hormones secreted by the pituitary gland, as well as the levels of hormones secreted by the target gland, must be evaluated to determine the source of an endocrine disorder. In some patients, an excessive amount of hormone may arise from an ectopic (outside) source, such as a bronchogenic cancer, rather than from a gland. In such cases, the levels of tropic hormones are low.
Blood tests are commonly used to check serum hormone levels, frequently making use of radioimmunoassay methods or, more recently, immunochemical methods (enzyme-multiplied immunoassay technique or chemoluminescence). The effectiveness of a hormone can be measured; for example, blood glucose or blood calcium levels may reflect the activity of the respective hormones. Twenty-four-hour urine tests are helpful for ascertaining daily levels of hormones or their metabolites rather than using a random level taken at a specific moment. Stimulation or suppression tests can be performed to confirm the hyperfunction or hypofunction of a gland.
Scans, ultrasound, and magnetic resonance imaging are also helpful for checking the location and type of lesion that may be present. Biopsy is essential to eliminate the possibility of malignancy.
Treatment
Treatment depends on the cause of the problem. Hormone deficits may be treated with replacement therapy; for example, insulin to treat diabetes mellitus.
Adenomas causing excessive secretions may be removed surgically or by radiation therapy. Removal may be essential when pressure from the mass causes additional problems. For example, pituitary tumors cause pressure inside the skull, compressing brain tissue.
Insulin and Diabetes Mellitus
Diabetes mellitus is caused by a relative deficit of insulin secretion from the beta cells in the islets of Langerhans or by the lack of response by cells to insulin (insulin resistance). To simplify the text, insulin deficit is used to cover both decreased secretion of the hormone and insulin resistance.
Insulin is an anabolic hormone (building up or synthesis of complex substances from simple molecules). Deficient insulin results in abnormal carbohydrate, protein, and fat metabolism because the transport of glucose and amino acids into cells is impaired, as well as the synthesis of protein and glycogen. In turn, these metabolic abnormalities affect lipid metabolism. Many tissues and organs in the body are adversely affected by diabetes.
Some types of cells are not affected directly by the deficit of insulin. Insulin is not required for the transport of glucose into brain cells. This is fortunate, because neurons require glucose constantly as an energy source. In the digestive tract, insulin is not required for glucose absorption. Exercising skeletal muscle can utilize glucose without proportionate amounts of insulin. This can be significant because excessive exercise can deplete blood glucose and result in hypoglycemia. Conversely, exercise is helpful in controlling blood glucose levels in the presence of an insulin deficit.
Type 1 and Type 2 Diabetes
There are two basic types of diabetes, Types 1 and 2 (Table 16-2). The classification system has been revised to better reflect the pathology. Type 1, formerly insulin-dependent diabetes mellitus, type I, or juvenile diabetes, is the more severe form. It occurs more frequently in children and adolescents, but can develop at any age. Although there is a genetic factor in the development of the disease, the insulin deficit results from destruction of the pancreatic beta cells in an autoimmune reaction, resulting in an absolute deficit of insulin in the body and therefore requiring replacement therapy. The amount of insulin required is equivalent to the metabolic needs of the body based on dietary intake and metabolic activity. Acute complications such as hypoglycemia or ketoacidosis are more likely to occur in this group. About 1 in every 400 to 500 children has Type 1 diabetes; Type 1 diabetes occurs in approximately 10% of all individuals diagnosed with diabetes. It is a major factor predisposing to strokes (cerebrovascular accident), heart attacks (myocardial infarction, peripheral vascular disease and amputation, kidney failure, and blindness.
TABLE 16-2
General Comparison of Type 1 and Type 2 Diabetes
| Type 1 | Type 2 | |
| Age at onset | Children and adults | Older but also younger adults |
| Onset | Acute | Insidious |
| Etiology | Autoimmune destruction | Familial, lifestyle and environmental factors, obesity |
| Family history | ||
| Body weight | Thin | Obese |
| Plasma insulin level | Very low | Decreased or normal |
| Treatment | Insulin replacement | Diet and exercise or oral hypoglycemic agents or insulin replacement |
| Occurrence of hypoglycemia or ketoacidosis | Frequent | Less common |
Type 2 diabetes, formerly referred to as non–insulin-dependent diabetes mellitus, Type II, or mature-onset diabetes, is based on decreased effectiveness of insulin or a relative deficit of insulin. This abnormality may involve decreased pancreatic beta cell production of insulin, increased resistance by body cells to insulin, increased production of glucose by the liver, or a combination of these factors.
This form of diabetes may be controlled by adjusting the need for insulin by:
• Increase use of glucose, such as with exercise
• Stimulating the beta cells of the pancreas to produce more insulin
Type 2 is a milder form of diabetes, often developing gradually in older adults, the majority of whom are overweight. However, there has been an increased incidence in adolescents and younger adults who are identified with metabolic syndrome, a complex of several pathophysiologic conditions marked by obesity, cardiovascular changes, and significant insulin resistance due to increased adipose tissue (see Chapter 23). Individuals with metabolic syndrome often have developed vascular or other chronic complications before diagnosis. A major concern at this time is the rapid climb in incidence of Type 2 diabetes, with prevalence now estimated at about 9% (18 million) of the population greater than 20 years of age. With increasing obesity seen in the population, it is anticipated that future incidence will increase significantly. Also, it is thought that there may be one undiagnosed case for every two to three diagnosed cases. Prevalence of Type 2 diabetes increases with age, with approximately half the cases found in persons greater than 55 years of age. There is a higher prevalence in African Americans, Hispanic Americans, and Native Americans.
Gestational diabetes may develop during pregnancy and disappear after delivery of the child (see Chapter 22). Approximately 5% to 10% of women who have gestational diabetes develop Type 2 diabetes some years later. A number of other types of diabetes and glucose intolerance vary in cause and severity. The following discussion focuses on Types 1 and 2.
A number of other types of diabetes are recognized and include:
• Prediabetes—an early manifestation of Type 2 diabetes
• Latent autoimmune diabetes in adults—a slow onset Type 1 autoimmune diabetes
• Maturity onset diabetes of the young—a rare form caused by a mutation in an autosomal dominant gene
Pathophysiology
An insulin deficit leads to the following sequence of events.
Initial Stage
1. Insulin deficit results in decreased transportation and use of glucose in many cells of the body.
2. Blood glucose levels rise (hyperglycemia).
5. Fluid loss through the urine and high blood glucose levels draw water from the cells, resulting in dehydration (see Chapter 2).
6. Dehydration causes thirst (polydipsia).
7. Lack of nutrients entering the cells stimulates appetite (polyphagia).
Progressive Effects.
If the insulin deficit is severe or prolonged, the process continues to develop, resulting in additional consequences, ultimately, diabetic ketoacidosis. This occurs more frequently in persons with Type 1 diabetes.
8. Lack of glucose in cells results in catabolism of fats and proteins, leading to excessive amounts of fatty acids and their metabolites, known as ketones or ketoacids, in the blood.
Ketones consist of acetone and two organic acids—beta-hydroxybutyric acid and acetoacetic acid. Because the liver and other cells are limited in the amount of lipids, fatty acids, or ketones they can process completely within a given time, excessive amounts of ketones in the blood cause ketoacidosis.
The ketoacids bind with bicarbonate buffer in the blood, leading to decreased serum bicarbonate and eventually to a decrease in the pH of body fluids. (Note that ketones can also accumulate in people on starvation diets.)
Signs and Symptoms
As Type 2 diabetes develops weight gain or increased abdominal girth is common, whereas in Type 1 weight loss is common. As blood glucose rises in the early stage, fluid loss is significant. Polyuria is indicated by urinary frequency, which is often noticed by the patient at night (nocturia) with the excretion of large volumes of urine. Thirst and dry mouth occur in response to fluid loss. Fatigue and lethargy develop. Weight loss may follow. Appetite is increased. Typically, the three Ps—polyuria, polydipsia, and polyphagia—herald the onset of diabetes. If the insulin deficit continues, the patient progresses to the stage of diabetic ketoacidosis.
Diagnostic Tests
Fasting blood glucose level, the glucose tolerance test, and the glycosylated hemoglobin (HbA1c) test are used to screen people with clinical and subclinical diabetes. There is less emphasis now on the “prediabetic stage” because tissue and organ damage appear to commence at an early stage. At present, a fasting blood sugar equal to or greater than 126 mg/dL, taken on more than one occasion, confirms a diagnosis of diabetes.
The test for HbA1c is used to monitor long-term control (8 to 12 weeks) of blood glucose levels. The test should be repeated every 3 months. The acceptable level for HbA1c has been lowered to 7%, and is likely to be lowered again to 6% (normal), so as to reduce the serious long-term effects of hyperglycemia.
Patients with diabetes can monitor themselves at home by taking a sample of capillary blood from a finger and checking it with a portable monitoring machine (glucometer). When performed regularly, this self-monitoring test helps reduce the fluctuations in blood glucose levels and therefore the risk of complications. Urine tests for ketones are helpful for those who are predisposed to ketoacidosis. Arterial blood gas analysis is required if ketoacidosis develops. Serum electrolytes may be checked as well.
Treatment
Maintenance of normal blood glucose levels is important to minimize the complications of diabetes mellitus, both acute and chronic. Glucose intake must be balanced with use. Treatment measures depend on the severity of the insulin deficit and may change over time. There are essentially three levels of control:
Diet.
Therapy is based on maintaining optimum body weight (weight reduction may be necessary) as well as control of blood glucose levels. This is important for persons with both types of diabetes. Recommended diets include more complex carbohydrates with a low glycemic index in contrast to simple sugars, which have a high glycemic index and elevate blood glucose rapidly; adequate protein; as well as maintaining low cholesterol and low lipid levels. Increased fiber with meals appears to reduce surges in blood sugar associated with food intake.
The total amount of food intake, as well as the distribution of the constituents, is important. Food intake must match available insulin and metabolic needs, including activity level. Various methods of meal planning are available from the diabetic associations and local diabetic clinics to ensure that the patient ingests a good balance of the various nutrients and provide information on the exchange of food components without disruption of goals. Nutritionists can be consulted on an individual basis in many diabetic clinics.