Chapter 3 Endocrine Physiology
I. Hormones
A. Overview
1. The primary function of hormones is to maintain homeostasis (e.g., regulate plasma glucose and electrolyte balance) and coordinate physiologic processes such as development, metabolism, and reproduction.
3. Hormones maintain homeostasis by using various feedback mechanisms.
| Hormones | Physiologic Actions | Pathophysiology |
|---|---|---|
| Hypothalamic Hormones | ||
| Corticotropin-releasing hormone (CRH) | Stimulates adrenocorticotropic hormone (ACTH) secretion from anterior pituitary | Increase in adrenal insufficiency due to loss of negative feedback |
| Gonadotropin-releasing hormone (GnRH) | Stimulates gonadotropin secretion from anterior pituitary | |
| Thyrotropin-releasing hormone (TRH) | Stimulates thyroid-stimulating hormone (TSH) secretion from anterior pituitary | Decrease in primary or secondary hyperthyroidism due to feedback inhibition |
| Growth hormone-releasing hormone (GHRH) | Stimulates growth hormone secretion from anterior pituitary | Decrease in growth hormone (GH)-secreting tumor of anterior pituitary |
| Somatostatin | Inhibits GH secretion from anterior pituitary | Synthetic version (octreotide) used in GH-secreting pituitary adenomas |
| Dopamine | Inhibits prolactin secretion from anterior pituitary | |
| Anterior Pituitary Hormones | ||
| Adrenocorticotropic harmone (ACTH) | Stimulates glucocorticoid and androgen synthesis in adrenal medulla | |
| Thyroid stimulating harmone (TSH) | Stimulates thyroid hormone synthesis in the thyroid gland | |
| Luteinizing hormone (LH) | Elevated levels: polycystic ovary syndrome (PCOS), testicular failure, premature menopause, Turner syndrome | |
| Follicle-stimulating hormone (FSH) | ||
| Growth hormone (GH) | Anabolic hormone with multiple anabolic and insulin-antagonizing metabolic effects | |
| Prolactin | Stimulates breast maturation and milk letdown | Prolactinoma: hypersecreting prolactinoma resulting in galactorrhea and infertility in women |
| Posterior Pituitary Hormones | ||
| Antidiuretic hormone (ADH) | Stimulates water absorption from the distal nephron | |
| Oxytocin | Stimulates uterine contraction during labor | |
| Thyroid Hormones | ||
| Thyroxine (T4) | Prohormone that becomes bioactive on peripheral conversion to T3 | Thyrotoxicosis: any cause for ↑ thyroid hormones (e.g., gland destruction, exogenous intake, ↑ synthesis) Thyroiditis: destruction of thyroid gland; can transiently cause hyperthyroidism but ultimately causes hypothyroidism |
| Triiodothyronine (T3) | Increases basal metabolic rate by up-regulating expression and insertion of Na+,K+-ATPase pump | |
| Adrenal Cortex Hormones | ||
| Aldosterone | Promotes renal Na retention and expands plasma volume | Hypersecreted in primary aldosteronism → hypertension with hypokalemic metabolic alkalosis |
| Cortisol | Helps maintain glucose for glucose-dependent tissues during fasting state by promoting hepatic gluconeogenesis, peripheral resistance to insulin, and lipolysis in adipose tissue | |
| Dehydroepiandrosterone (DHEA) | Converted to testosterone in peripheral tissues | Congenital adrenal hyperplasia: oversecretion of androgens results in virilization, precocious puberty, ambiguous genitalia |
| Adrenal Medulla Hormones | ||
| Epinephrine | ||
| Ovarian Hormones | ||
| Estrogen | ||
| Testicular Hormones | ||
| Testosterone | ||
| Dihydrotestosterone (DHT) | Development of the male external genitalia (penis, scrotum) and prostate gland | |
| Pancreatic Hormones | ||
| Insulin | Promotes peripheral uptake of glucose in nonfasting (fed) state | |
| Glucagon | Promotes hyperglycemia and insulin resistance | Glucagonoma |
| Somatostatin | Used to treat GH-secreting pituitary adenomas | |
| Vasoactive-intestinal peptide (VIP) | Vasoactive intestinal polypeptide-secreting tumor (VIPoma); may be associated with multiple endocrine neoplasia type 1 | |
B. Mechanism of action of hormones
1. All hormones must interact with a cellular receptor, which then transduces a signal and generates a cellular response.
2. The effectiveness of a given hormone, therefore, depends on the concentration of free hormone (that which is available for binding), the concentration of hormone receptor, and the effectiveness of the transduction mechanism.
C. Types of hormones and their individual effector mechanisms
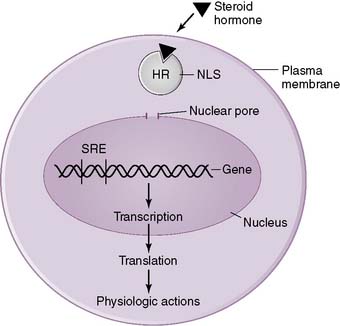
1. Steroid hormones
• Steroid hormones are lipid-soluble compounds derived from cholesterol that are able to enter all cells of the body by diffusing through the lipid-rich plasma membrane.
• They produce their effects by binding to receptors in either the cytosol or nucleus of cells in target tissues, and this hormone-receptor complex then activates transcription of specific genes (Fig. 3-2).
• Because steroid hormones can diffuse freely through lipid membranes, they cannot be stored within intracellular vesicles.
• Furthermore, because steroid hormones are lipid soluble, they must circulate bound to plasma proteins.
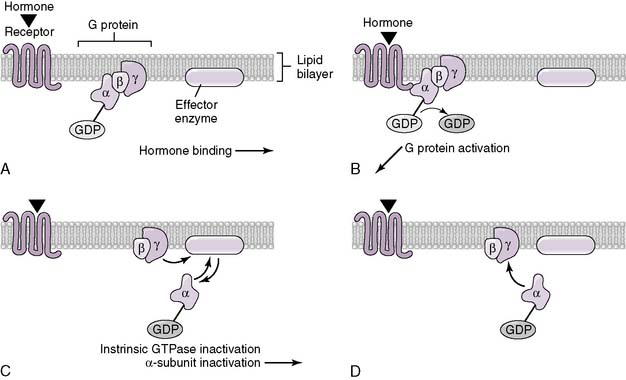
3. Proteoglycan, protein, peptide, and amino acid hormones
• Because they are hydrophilic, they can be stored in cytoplasmic vesicles within endocrine cells and released on demand.
• Some travel free as soluble compounds in the blood, whereas others travel mainly bound, associated with specific binding proteins.
• There are four primary classes of membrane-spanning receptors to which these hormones can bind:
D. Hormone-binding proteins
2. These binding proteins serve several important physiologic functions:
• They provide a reservoir of hormone, which exists in equilibrium with the free hormone and buffers any moment-to-moment changes in free hormone concentration.
3. All steroid hormones and a few peptide hormones have plasma binding proteins (Table 3-2).
TABLE 3-2 Hormone Binding in Some Plasma Proteins
| Plasma Protein | Hormone |
|---|---|
| Albumin | Multiple lipophilic hormones |
| Transthyretin | Thyroxine (T4) |
| Transcortin | Cortisol, aldosterone |
| Thyroxine-binding globulin | Triiodothyronine (T3), T4 |
| Sex hormone–binding globulin | Testosterone, estrogen |
E. Hierarchical control of hormone secretion
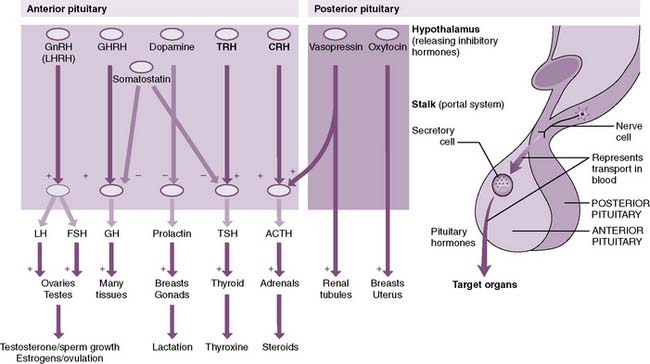
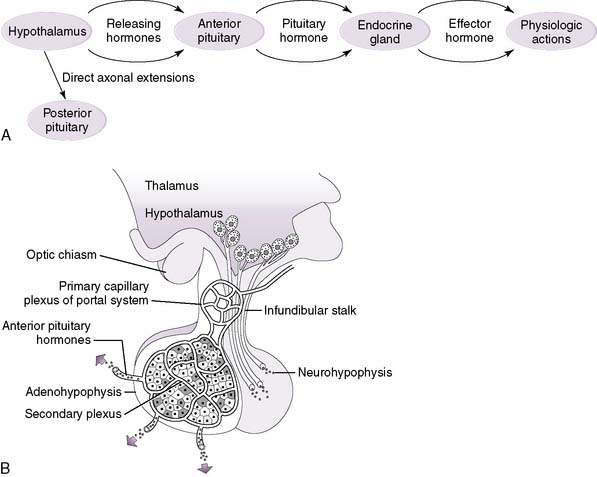
1. For several hormonal control systems, a hierarchical axis exists, consisting of the hypothalamus, the anterior pituitary (adenohypophysis), and a specific endocrine gland (Fig. 3-4A).
2. The hypothalamus, at the top of the axis, secretes releasing (and inhibitory) hormones into a capillary bed that converges on the pituitary and then re-expands into another capillary bed within the anterior pituitary (hypothalamic-hypophyseal portal system) (Table 3-3; Fig. 3-4B).
TABLE 3-3 Effect of Hormones Released by the Hypothalamus on the Anterior Pituitary
| Hormone | Effect on Anterior Pituitary |
|---|---|
| Growth hormone–releasing hormone (GHRH) | Stimulates growth hormone (GH) secretion |
| Prolactin-inhibitory factor (dopamine) | Inhibits prolactin secretion |
| Somatostatin | Inhibits GH secretion |
| Gonadotropin-releasing hormone (GnRH) | Stimulates luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion |
| Corticotropin-releasing hormone (CRH) | Stimulates adrenocorticotropic hormone (ACTH) secretion |
| Thyrotropin-releasing hormone (TRH) | Stimulates thyroid-stimulating hormone (TSH) secretion |
3. The releasing hormones then stimulate specific cell types of the anterior pituitary and stimulate (or inhibit) pituitary hormone secretion.
4. The pituitary hormone, in turn, may act directly on target tissues (e.g., prolactin) or stimulate an endocrine gland to produce an effector hormone (e.g., thyroid-stimulating hormone).
5. The hypothalamus also controls the secretion of the hormones of the posterior pituitary (neurohypophysis), but in a different fashion.
6. Posterior pituitary hormones are synthesized by neurons in the hypothalamus and transported along axons into the posterior pituitary.
F. Classification of endocrine diseases
1. A hormone deficiency or excess can occur as the result of a defect anywhere along the hypothalamic-pituitary-target organ axis.
II. Hormonal Control Systems of the Anterior Pituitary

A. Hypothalamic-pituitary-adrenal axis
Get Clinical Tree app for offline access
1. Overview
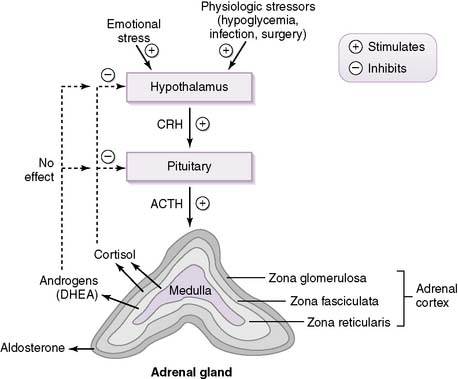
• Corticotropin-releasing hormone (CRH) from the hypothalamus stimulates the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary through activation of corticotroph cells.
• ACTH then acts on the adrenal cortex to stimulate the synthesis and secretion of glucocorticoids and androgens but not the mineralocorticoid aldosterone (see pathology note below).
2. Regulation of the hypothalamic-pituitary-adrenal axis
• As shown in Figure 3-5, cortisol secretion is stimulated by hypoglycemia or stressful conditions (e.g., surgery), when the sympathetic nervous system is also activated.
• Cortisol secretion is normally inhibited by increased plasma levels of cortisol because of the negative-feedback effect of cortisol on the pituitary and hypothalamus.
• Note from the figure that androgens do not inhibit CRH or ACTH secretion, as discussed in the pathology note above.
• Cortisol has a diurnal pattern of secretion that is based on the daily pattern of ACTH secretion from the pituitary.
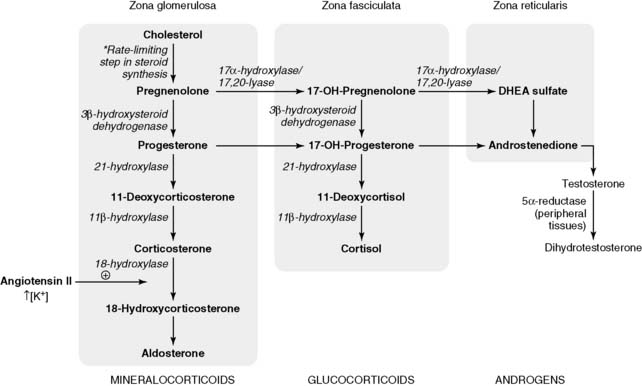
3. Biosynthetic pathway of adrenal corticosteroids
• The rate-limiting step in adrenal steroid synthesis is the conversion of cholesterol to pregnenolone (Fig. 3-7).
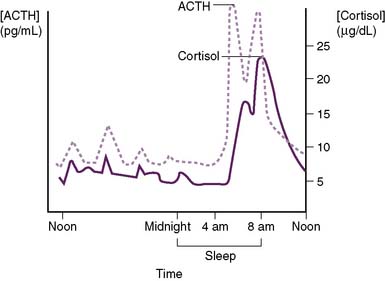

3-6 Diurnal secretion of adrenocorticotropic hormone and cortisol. ACTH, Adrenocorticotropic hormone.
• Although the principal mineralocorticoid aldosterone is synthesized in the adrenal cortex, its synthesis is only slightly affected by ACTH.
4. Mechanism of action of cortisol (see Fig. 3-1)
• As a steroid hormone, cortisol is able to diffuse through the plasma membrane of cells and bind to a cytoplasmic receptor.
• This hormone-receptor complex then enters the nucleus, binds specific DNA sequences, and regulates the expression of various “steroid-responsive” genes.
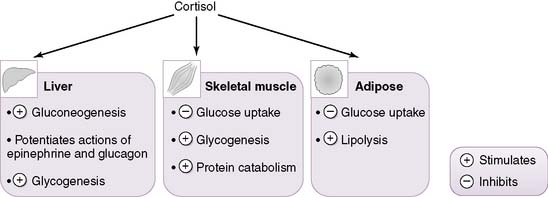
5. Physiologic actions of cortisol (Fig. 3-8)
• Fuel metabolism
a. In the fasting state, cortisol helps maintain adequate plasma levels of glucose for glucose-dependent tissues such as the central nervous system (CNS).
b. It accomplishes this by inhibiting the peripheral utilization of glucose by muscle and adipose tissue while simultaneously stimulating hepatic gluconeogenesis.
c. Cortisol exerts catabolic actions on most tissues, with the exception of the liver, on which it exerts anabolic actions.
d. Cortisol stimulates hepatic gluconeogenesis in several ways:
• It promotes muscle breakdown, which releases amino acids (e.g., alanine, aspartate) into the gluconeogenic pathway.
• Cortisol additionally stimulates lipolysis in adipose tissue, which helps maintain plasma levels of glycerol and fatty acids during the fasting state.
• These substrates can then be used as alternative fuel sources in various tissues (e.g., muscle), thereby sparing plasma glucose for the CNS.
• Effects on blood pressure and plasma volume
a. Cortisol increases blood pressure in several ways.
• It increases the expression of adrenergic receptors in various tissues.
(1) For example, stimulation of α1-adrenergic receptors on vascular smooth muscle results in vasoconstriction.
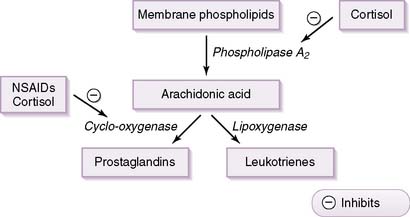
• Effects on inflammatory and immune responses
b. It inhibits activity of the enzyme phospholipase and also inhibits the transcription of various inflammatory cytokines.
c. As shown in Figure 3-9, inhibition of phospholipase leads to decreased arachidonic acid production and, therefore, to decreased production of prostaglandins and leukotrienes, both potent inflammatory mediators.
• Effects on bone
a. At supraphysiologic levels, cortisol weakens bones by inhibiting bone-forming cells (osteoblasts) and stimulating bone-degrading cells (osteoclasts).
5. Pathophysiology of the CRH-ACTH-cortisol axis
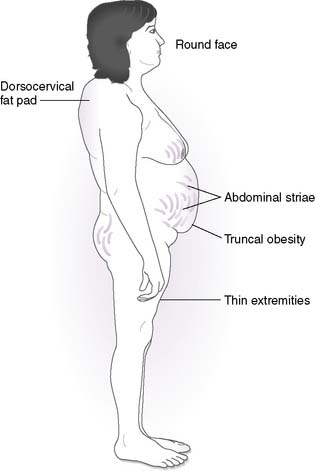
• Hypercortisolism (Cushing syndrome)
a. Cushing syndrome refers to the constellation of signs and symptoms associated with hypercortisolism, irrespective of the etiology of the hypercortisolism.
b. Recall that cortisol promotes hyperglycemia, muscle breakdown, bone loss, and plasma volume expansion.
d. The most common endogenous source of elevated cortisol is an ACTH-hypersecreting tumor of the pituitary (pituitary Cushing), a condition formerly referred to as Cushing disease.
e. Other causes of Cushing syndrome (Fig. 3-10) include cortisol-hypersecreting adrenal tumors and ectopic (paraneoplastic) production of ACTH by tumors (e.g., small cell lung cancer).
• Hypocortisolism (adrenal insufficiency)
a. Principal pathologic consequences include fatigue and vague abdominal pain, although additional manifestations are numerous and may include orthostatic hypotension, hypoglycemia, hyponatremia, and chronic diarrhea, to name just a few.
b. Most common cause is iatrogenic, because of the abrupt cessation of chronically administered steroids; in this case, the hypothalamic-pituitary-adrenal axis has been chronically suppressed and needs several weeks to “wake up.”
6. Hypothalamic-pituitary regulation of adrenal androgen synthesis
• Both substances are androgen prohormones that are converted in peripheral tissues to testosterone and dihydrotestosterone (DHT).
7. Pathophysiology of congenital adrenal hyperplasias (CAH)
• In CAH, decreased cortisol production disinhibits the pituitary, causing increased ACTH secretion.
• Continued stimulation by ACTH leads to shunting of cortisol precursors to androgens, which may cause precocious puberty in males later in childhood or ambiguous genitalia in female neonates.
• The most common form of CAH is caused by 21-hydroxylase deficiency (Fig. 3-11).