Electron Microscopy
Gary W. Mierau, Ph.D.
Eric P. Wartchow, Ph.D.(c)
M. John Hicks, M.D., D.D.S., M.S., Ph.D.
Electron microscopy remains an essential tool for today’s pediatric pathologist. Although now increasingly being used as a means to refine and guide the course of more costly and narrowly specialized diagnostic studies, the technique continues to provide for a significant number of childhood diseases the best and sometimes only means of establishing a definitive diagnosis. Offering a direct and open morphologic approach, and not being confined to hypothesis testing, it is one of the more powerful and least treacherous ancillary diagnostic techniques currently available. This having been said, it is also recognized that each special technique has its relative strengths and weaknesses in particular situations. These should be regarded as complementary tools, which are best employed using a highly selective but fully integrated approach.
We have found ancillary electron microscopic studies to be warranted in the workup of approximately five percent of surgical specimens submitted for histologic diagnosis. In contrast to the situation with adult patients, where renal specimens predominate, a very broad mix of specimens is received from pediatric patients (Figure 1D-1). Respiratory tract specimens for the evaluation of cilia morphology comprise the largest proportion of diagnostic cases, followed by liver, kidney, and muscle, and then in gradually diminishing numbers by a wide variety of other tissue types.
In addition to the more customary applications in diagnostic surgical and autopsy pathology, electron microscopy can play a valuable role in a departmental quality assurance program for example through the examination of a sampling of tumor specimens for which such studies were not initially considered necessary for diagnostic purposes. Our experience has been that this practice will lead to a reconsideration of the diagnosis in a significant number of cases.
A number of institutions are utilizing electron microscopy in the clinical laboratory as a primary means of detecting virus in diarrheal stool specimens, blood, skin vesicles, and other bodily fluids. The development of alternative technologies, such as multiplex PCR, may in time come to diminish its role in this context. Even if this does happen, this technology will remain essential for the detection of new or unusual agents.
Opportunities currently abound also for the use of electron microscopy in pathology research, in particular where integration or correlation of nonstructural with structural information is of benefit.
THE ELECTRON MICROSCOPY LABORATORY
The central element in any electron microscopy laboratory is, of course, the electron microscope itself. A basic transmission electron microscope is all that is needed for diagnostic applications (Figure 1D-2). Ease of operation, reliability, and optimal performance at low magnifications are of far greater importance in this setting than are the advanced features offered by the more expensive and more demanding “analytical” microscopes. Electron microscopes have achieved a relatively mature state of technologic development, and several manufacturers produce excellent (100 to 120 kV) instruments suitable for this application. In contrast to the situation with respect to the electron microscopes themselves, the technology associated with image capture, recording, transmission, and storage has recently undergone revolutionary changes. Digital cameras offer many advantages and have now largely replaced film cameras. Coupled with an Internet-based computerized delivery system, they enable a very significant reduction in turnaround time with relatively little compromise to quality. Other than the electron microscope, the only specialized equipment needed is an ultramicrotome. Because ultimate success with this technique depends so heavily upon the capacity to obtain quality ultrathin sections in a reliable and rapid manner, it is of greater importance to possess a modern ultramicrotome than a modern electron microscope.
A scanning electron microscope can occasionally be used to advantage (e.g., for demonstration of hair shaft abnormalities) but is not a necessity for a basic facility. Similarly, the
capacity to perform elemental analysis (e.g., for heavy metal detection) or tomographic studies (e.g., for problematic cilia specimens) is a need only for larger more sophisticated referral laboratories.
capacity to perform elemental analysis (e.g., for heavy metal detection) or tomographic studies (e.g., for problematic cilia specimens) is a need only for larger more sophisticated referral laboratories.
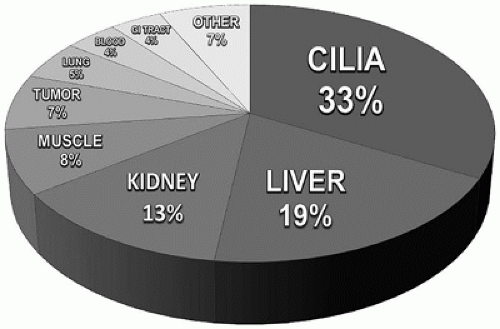 FIGURE 1D-1 • Composition of pediatric cases submitted for diagnostic ultrastructural study (Children’s Hospital Colorado, 2013, n = 603). |
Generating results within a clinically relevant time frame is crucial to a successful operation, and it is a reasonable expectation to have an interpretive report, complete with illustrations, available within two working days of specimen receipt. Even “same day” examination of specimens is possible when circumstances warrant. One dedicated technologist for every 250 specimens examined annually continues to be a reasonable guide for the staffing of a diagnostic EM facility (1). To ensure a steady and secure service, two technologists and therefore a minimum caseload of approximately 500 specimens per year are needed. Institutions with fewer specimens may be better served by outsourcing, at least the technical component of this work, to a provider that can consistently meet this standard. Overnight delivery systems and electronic data transmission mechanisms obviate any need for the EM provider to be in close proximity to the referring institution.
In providing the interpretive component of the ultra-structural studies, a number of organizational models have been shown effective. Which is best in a given situation will depend upon the particular circumstances and personnel available. In some institutions, each pathologist while on service assumes full responsibility for the submission, examination, and interpretation of their cases. In others, a designated pathologist carries the responsibility for the examination and interpretation of all cases. In the majority of laboratories, however, technical personnel (with appropriate training and guidance) do much of the examination and may even assist with the interpretation of results.
It is often assumed that the electron microscopy laboratory will be a financial liability for its parent institution. This need not be true. With attentiveness to basic business practices, and sufficient volume, an electron microscopy laboratory can be a profitable enterprise. Electron microscopy is sometimes thought of as being a very expensive and extremely slow technique, but its modern-day cost and speed is actually quite comparable to immunohistochemistry and most other ancillary diagnostic techniques. And, of course, the cost savings to be derived from utilizing the most powerful techniques available to obtain a fast and accurate diagnosis needed to minimize the length of a hospital stay should be obvious. Certainly for health care facilities already maintaining an electron microscopy laboratory, there is no economic reason not to use the technique to its fullest advantage. The major expenses associated with this endeavor are fixed rather than incremental, so its actual cost to the institution will remain virtually the same whether it is used a little or used a lot.
THE ELECTRON MICROSCOPY TECHNIQUE
While most other ancillary techniques (e.g., IHC, FISH, PCR) are restricted in application to hypothesis testing, electron microscopy can provide the right answer even when the wrong question, or no specific question,
is being asked. Preserving the option to perform electron microscopy is therefore an important habit to develop. Placing a bit of tissue into an appropriate fixative as a matter of routine takes little extra time and costs next to nothing— but provides excellent insurance should a diagnostic issue arise later.
is being asked. Preserving the option to perform electron microscopy is therefore an important habit to develop. Placing a bit of tissue into an appropriate fixative as a matter of routine takes little extra time and costs next to nothing— but provides excellent insurance should a diagnostic issue arise later.
Electron microscopy is not so much a different technology as a modification, and extension, of a most familiar one. Specimens for electron microscopy are handled in almost the same way as for light microscopy. Here, the standard fixative, instead of formaldehyde, is glutaraldehyde (to better preserve proteins) followed by osmium tetroxide (to better preserve lipids). Ordinary formalin preparations, if they are properly buffered (pH 7.2-7.4 range) and adjusted to moderate hypertonicity (400 to 500 mOsm), actually serve quite well as a primary fixative for electron microscopy and can be substituted when necessary. The tissues are dehydrated in a similar fashion but then are embedded in an epoxy resin to enable the cutting of thinner sections than would be possible with the softer paraffin wax used for routine histology. To cut the necessary (approximately 80 nm thick) ultrathin sections requires a similar but more refined “ultra” microtome and the use of a diamond blade. An electron beam cannot penetrate glass, so the sections are mounted on a fine-meshed screen (referred to as a grid) rather than on a glass slide. The sections are not stained in the true sense of the word (as color reactions cannot be detected with an electron microscope) but are incubated in similar fashion in heavy metal solutions (usually of uranium and lead) to selectively add contrast to various substructural components. Methodologic details for all these procedures can be found in many standard texts (2, 3). Except in its use of a beam of electrons, rather than a beam of light, the electron microscope is not very different in design or operation from that of an ordinary light microscope. It is the shorter wavelength of electrons that enables the superior point-to-point resolution and, thus, higher working magnifications offered by this instrumentation. The two techniques form a strong partnership, with the light microscope being best suited for the study of collections of cells and the electron microscope being best suited for the study of individual cells.
Under ideal conditions, specimens submitted for electron microscopy will consist of a representative sampling of appropriately sized (approximately 1 mm3) tissue cubes placed into the most suitable fixative immediately upon removal from the patient. Real-life conditions will not always be ideal, but all is not necessarily lost if they are not. Just as an experienced automobile mechanic can still usually identify the make and model of a car after it has been involved in an accident, so can an experienced electron microscopist still usually identify the cell type and disease process involved in a partially wrecked tissue specimen. One has to be more cautious when dealing with suboptimal specimens, however, as there is a strong inverse relationship between the quality of specimen preservation and the probability of making a significant interpretive error. The safest strategy when dealing with suboptimally preserved specimens is to restrict electron microscopy to the search for some particular feature(s) predetermined to be of diagnostic relevance.
A demonstration of the deleterious effects associated with suboptimal specimen processing is presented in Figure 1D-3, which shows subsamples from the same case of Langerhans cell histiocytosis after being subjected to progressively harsher treatments. Here, it can be seen that, though the diagnosis can still be made, the degree of difficulty increases (and the degree of confidence decreases) as the quality of cellular preservation is diminished. With optimal processing (Figure 1D-3A), the richness of cytoplasmic detail almost obscures the diagnostic Birbeck granules. Substitution of formaldehyde for glutaraldehyde as the primary fixative (Figure 1D-3B) results in a significant loss of cytoplasmic detail, but, at least in this instance, this does not interfere with the identification of the critical feature. Often, it is the case that in order to perform additional specialized procedures, such as the immunohistochemical reaction for S100 protein shown here, it becomes necessary to trade off some degree of cellular preservation to maintain an adequate degree of tissue reactivity. Such applications, however, will fall mainly within the domain of research. For general purposes, when using formalin-fixed tissues for electron microscopy, it is best to refix the specimen in glutaraldehyde before proceeding with the tissue processing. As a last resort, one can retrieve paraffin-embedded tissue and reprocess it for electron microscopy. The technique is simple (4). Following removal of a carefully selected appropriately sized tissue sample from the paraffin block using a sharply pointed scalpel blade, the specimen is dewaxed overnight in xylene. Best results are obtained if, following rehydration in graded alcohols, the tissue is refixed both with glutaraldehyde and with osmium tetroxide prior to further processing. Because much of the lipid will have been extracted during the earlier processing events, one can expect poor preservation of membranes and other structures of high lipid content. Nevertheless, as shown in Figure 1D-3C, the features of key interest may still remain clearly identifiable. Structures that are composed largely of proteins (e.g., filaments, granules, intercellular junctions, immune deposits) are the most likely to remain identifiable, but sometimes, membranous structures also are preserved. It proved possible, for example, in a correlative study to demonstrate Birbeck granules in deparaffinized material from 11 of 14 cases in which they were known to exist (5). In some situations, for example, in attempting to identify a focally distributed virus, this approach may actually prove more efficacious than would an unfocused search through optimally preserved tissue. Utilization of deparaffinized tissue preserved with a nonaldehyde-type fixative (e.g., alcohol, B5, Bouin) will generally be unrewarding for, without cross-linking of proteins, nearly all substructural features are lost during processing (Figure 1D-3D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



