Chapter 52 Drugs to Treat Parasitic Infections
| Abbreviations | |
|---|---|
| AIDS | Acquired immunodeficiency syndrome |
| CNS | Central nervous system |
| DNA | Deoxyribonucleic acid |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GI | Gastrointestinal |
| IM | Intramuscular |
| IV | Intravenous |
Therapeutic Overview
CLASSIFICATION OF MAJOR PARASITIC GROUPS
There are two major groups of parasites: multicellular helminths (worms) and single-celled protozoa.
| Therapeutic Overview |
|---|
| Prevention Strategies |
| Control disease vectors or reduce contact with them |
| Improve hygiene and sanitation |
| Vaccine development |
| Drugs |
| Transmission of Protozoal Infection |
| Malaria—mosquitoes |
| Leishmaniasis—sand flies |
| African trypanosomiasis—tsetse flies |
| Chagas’ disease—reduviid bugs |
| Amebiasis—food, water |
| Giardiasis—food, water |
| Toxoplasmosis—cats, undercooked meats |
Helminths have sophisticated organ systems and many have complex life cycles. Clinical manifestations of helminthic diseases are usually proportionate to the worm burden. Infections with light worm burdens are often asymptomatic, whereas heavy worm burdens can result in life-threatening disease. Exceptions occur when one or more helminths gain access to a critical organ such as the brain or an eye, or when an adult worm migrates into and obstructs the common bile duct, such as with A. lumbricoides. Helminths have finite life spans. Infestations resolve over time, unless there is autoinfection, as in the case of Strongyloides stercoralis or Hymenolepis nana, or the parasite has an extremely long life span, as in the case of Clonorchis sinensis. Eosinophilia is common when helminths migrate through tissue but may be absent after intestinal helminths have reached maturity in the bowel lumen.
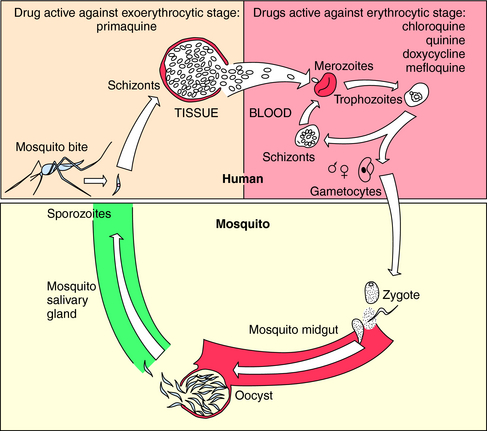
The vectors by which parasites spread are varied. Enteric pathogens are spread in fecally contaminated food and water, T. vaginalis is spread by intimate personal contact, whereas Plasmodium species, which cause malaria, are transmitted by anopheline mosquitoes, whose life cycle is depicted in Figure 52-1. Sporozoites are inoculated into the host when an infected female attempts to take a blood meal. The sporozoites travel to the liver through the circulation, invade hepatocytes, and develop within liver cells in 1 to 3 weeks. The erythrocytic stage, which is the only symptomatic stage, begins when merozoites are released from the liver and invade red blood cells. Plasmodium vivax and Plasmodium ovale, in contrast, can persist for months in the liver as hypnozoites before completing development and initiating symptomatic malaria.
Mechanisms of Action
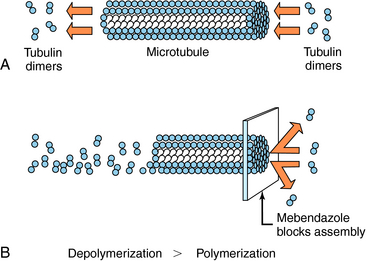
Albendazole sulfoxide, the primary metabolite of albendazole, and mebendazole bind to β-tubulin in susceptible nematodes and inhibit microtubule assembly, leading to disruption of microtubules and selective and irreversible inhibition of glucose uptake (Fig. 52-2). This results in depletion of glycogen stores, reduced formation of adenosine triphosphate, disruption of metabolic pathways, and ultimately parasitic death. Serum glucose concentrations are not affected in the human host.
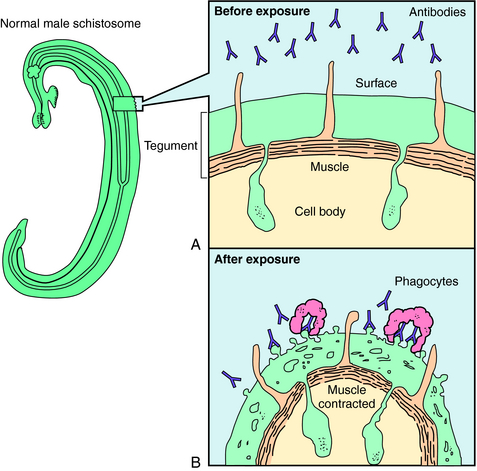
Praziquantel is a heterocyclic pyrazine-isoquinoline derivative. It is rapidly taken up by tapeworms and flukes, but its precise mechanism of action is not known. Studies of the tapeworm Hymenolepis diminuta indicate that praziquantel releases Ca++ from endogenous stores, resulting in contraction and subsequent expulsion of the worm from the GI tract. In the schistosomes, praziquantel damages the tegument, causing intense vacuolation, exposure of sequestered schistosomal antigens, and increased permeability to Ca++, causing tetanic contraction and paralysis. Adult schistosomes are then swept back through the portal circulation to the liver, where they are destroyed by phagocytes. Figure 52-3 depicts the marked alterations in the schistosomal surface after drug exposure.
A summary of the observed effects and possible mechanisms of action of the major antihelminthic drugs is in Table 52-1.
TABLE 52–1 Observed Effects and Possible Mechanisms of Action of the Major Anthelmintic Drugs
| Drug | Observed Effects on Helminths | Possible Mechanism of Action |
|---|---|---|
| Albendazole | Inhibition of glucose transport; depletion of glycogen stores, inhibition of fumarate reductase | Binding to β-tubulin, prevents microtubule polymerization |
| Mebendazole | Inhibition of glucose transport; depletion of glycogen stores | Binding to β-tubulin |
| Pyrantel pamoate | Muscles depolarize, increased spike wave activity, spastic paralysis | Depolarizing neuromuscular blockade |
| Diethylcarbamazine | Hyperpolarization and paralysis of worm’s musculature; exposure of antigens, leading to antibody binding and attack by phagocytes | Hyperpolarization and neuromuscular blockade |
| Ivermectin | Alters chloride currents, resulting in death of microfilariae | Altered chloride channel function |
| Praziquantel | Depolarization of muscles, increased intracellular Ca++, displacement of schistosomes to the human liver, exposure of surface antigens, binding by antibody and phagocytes, tegument disruption | Uncertain |
| Niclosamide | Uncouples phosphorylation; may inhibit anaerobic metabolism | Uncertain |
Metronidazole has a broad spectrum of activity against anaerobic bacteria and protozoa. It is activated when reduced by ferredoxins or their equivalents in protozoa or bacteria. The resultant products react with deoxyribonucleic acid (DNA) and other intracellular parasite constituents, causing damage and death. Tinidazole has a similar mechanism of action. Paromomycin, an aminoglycoside antibiotic (see Chapter 47), inhibits protein synthesis. Iodoquinol acts against E. histolytica cysts and, to a lesser extent, trophozoites by an unknown mechanism. Diloxanide furoate is directly amebicidal, and little is known about its mechanism of action also. Nitazoxanide has a broad spectrum of activity against protozoa and helminths and is approved for giardiasis and cryptosporidiosis in children. The mechanism involves inhibition of electron transport reactions essential to metabolism of anaerobic organisms. Furazolidone interferes with several bacterial enzyme systems, but its mechanism of action against G. lamblia is uncertain.
Atovaquone has activity against Plasmodium spp., Babesia spp., P. jiroveci, and T. gondii. It selectively inhibits electron transport, resulting in collapse of the mitochondrial membrane potential. It also inhibits pyrimidine biosynthesis, which is obligatorily coupled to electron transport in Plasmodium spp. Pyrimethamine binds to and irreversibly inhibits dihydrofolate reductase. It is approximately 1000-fold more active against plasmodium dihydrofolate reductase-thymidylate synthetase than against human dihydrofolate reductase. Pyrimethamine is often used with one of the sulfonamides to inhibit sequential steps in folate metabolism. Trimethoprim inhibits the dihydrofolate reductase of many bacteria and some protozoa and is frequently administered with sulfamethoxazole (see Chapter 48). Proguanil is metabolized to an active cyclic triazine metabolite that selectively inhibits plasmodium dihydrofolate reductase-thymidylate synthetase. Nifurtimox undergoes partial reduction followed by auto-oxidation, forming superoxide anion, hydrogen peroxide, and hydroxyl radicals that damage cell membranes and DNA. Eflornithine is an irreversible inhibitor of ornithine decarboxylase, the enzyme that catalyzes the rate-limiting step in polyamine synthesis. Although polyamines are essential for growth and differentiation of all cells, eflornithine has clinical activity only against T. brucei gambiense.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree