Fate
Thyroid hormones are released from the thyroid gland by a proteolytic process. The amount of T4 released is substantially greater than the amount of T3 released. However, much of the T4 that is released undergoes conversion to T3 by enzymes in peripheral tissues. In fact, conversion of T4 to T3 accounts for the majority (about 80%) of the T3 found in plasma.
More than 99.5% of the T3 and T4 in plasma is bound to plasma proteins. Consequently, only a tiny fraction of circulating thyroid hormone is free to produce biologic effects.
Thyroid hormones are eliminated primarily by hepatic metabolism. Because T3 and T4 are extensively bound to plasma proteins, metabolism is slow. As a result, the half-lives of these hormones are prolonged—about 1 day for T3 and 7 days for T4.
Thyroid Hormone Actions
Thyroid hormones have three principal actions: (1) stimulation of energy use, (2) stimulation of the heart, and (3) promotion of growth and development. Stimulation of energy use elevates the basal metabolic rate, resulting in increased oxygen consumption and increased heat production. Stimulation of the heart increases both the rate and force of contraction, resulting in increased cardiac output and increased oxygen demand. Thyroid effects on growth and development are profound: thyroid hormones are essential for normal development of the brain and other components of the nervous system, and they have a significant effect on maturation of skeletal muscle.
Thyroid hormones produce their effects by modulating the activity of specific genes. Furthermore, it appears that most, if not all, of the effects of thyroid hormones are mediated by T3, not by T4. There is good evidence that T3 penetrates to the cell nucleus and binds with high affinity to nuclear receptors, which in turn bind to specific DNA sequences. The result is modulation of gene transcription, causing production of proteins that mediate thyroid hormone effects. Although T4 also binds with nuclear receptors, its affinity is low, and gene transcription is not altered. Hence it would seem that T4 serves only as a source of T3, having little or no physiologic effects of its own.
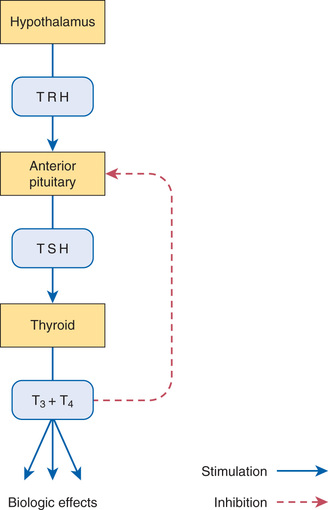
Regulation of Thyroid Function by the Hypothalamus and Anterior Pituitary
The functional relationship between the hypothalamus, anterior pituitary, and thyroid is shown in Fig. 47.2. As indicated, thyrotropin-releasing hormone (TRH), secreted by the hypothalamus, acts on the pituitary to cause secretion of thyrotropin (thyroid-stimulating hormone [TSH]). TSH then acts on the thyroid to stimulate all aspects of thyroid function: thyroid size is enlarged, iodine uptake is augmented, and synthesis and release of thyroid hormones are increased. In response to rising plasma levels of T3 and T4, further release of TSH is suppressed. The stimulatory effect of TSH on the thyroid, followed by the inhibitory effect of thyroid hormones on the pituitary, constitutes a negative feedback loop.
Effect of Iodine Deficiency on Thyroid Function
When iodine availability is diminished, production of thyroid hormones decreases. The ensuing drop in thyroid hormone levels promotes release of TSH, which acts on the thyroid to increase its size (causing goiter) and ability to concentrate iodine. If iodine deficiency is not too severe, the increased capacity for iodine uptake will restore normal production of T3 and T4.
Thyroid Function Tests
Several laboratory tests can be used to evaluate thyroid function. Three are described here. Values indicating euthyroid (normal), hypothyroid, and hyperthyroid states are shown in Table 47.1.
TABLE 47.1
Serum Values for Thyroid Function Tests*
| Thyroid Test | Serum Values | ||
| Normal | Hypothyroid | Hyperthyroid | |
| Total T4 (mcg/dL) | 4.5–12.5 | Under 4.5 | Over 12.5 |
| Free T4 (ng/dL) | 0.9–2 | Under 0.9 | Over 2 |
| Total T3 (ng/dL) | 80–220 | Under 80 | Over 220 |
| Free T3 (pg/dL) | 230–620 | Under 230 | Over 620 |
| TSH (microunits/mL) | 0.3–6 | Over 6 | Under 0.3 |
Serum Thyroid-Stimulating Hormone Test
Serum TSH determinations are used primarily for screening and diagnosis of hypothyroidism and for monitoring replacement therapy in hypothyroid patients.
Measurement of serum TSH is the most sensitive method for diagnosing hypothyroidism because the anterior pituitary is exquisitely sensitive to changes in thyroid hormone levels. As a result, very small reductions in serum T3 and T4 can cause a dramatic rise in serum TSH. Therefore even when the degree of hypothyroidism is minimal, it will be reflected by an abnormally high level of TSH. When replacement therapy is instituted, the TSH level should return to normal.
Serum TSH determinations can also be used to distinguish primary hypothyroidism from secondary hypothyroidism. In primary (thyroidal) hypothyroidism, TSH levels are high. However, in secondary hypothyroidism (hypothyroidism resulting from anterior pituitary dysfunction), TSH levels are low, normal, or even slightly elevated—despite the presence of low levels of T3 and T4.
Serum Thyroxine Test
Testing can measure either total T4 (bound plus free) or free T4. Measurement of free T4 is preferred. The T4 test can be used to monitor thyroid hormone replacement therapy and to screen for thyroid dysfunction. However, in both cases, measurement of TSH is preferred.
Serum Triiodothyronine Test
As with T4, we can measure either total or free T3. Measurement of free T3 is preferred. This test is useful for diagnosing hyperthyroidism. In this disorder, levels of T3 often rise sooner and to a greater extent than do levels of T4. T3 determinations can also be used to monitor thyroid hormone replacement therapy (all thyroid preparations should increase levels of T3).
Thyroid Pathophysiology
Hypothyroidism
Hypothyroidism can occur at any age. In adults, mild deficiency of thyroid hormone is referred to simply as hypothyroidism. Severe deficiency is called myxedema. When hypothyroidism occurs in infants, the resulting condition is called congenital hypothyroidism.
Hypothyroidism in Adults
Clinical Presentation
Signs and symptoms of hypothyroidism depend on disease severity. With mild hypothyroidism, symptoms are subtle and may go unrecognized for what they are. In contrast, with moderate to severe disease, characteristic signs and symptoms emerge. The face is pale, puffy, and expressionless. The skin is cold and dry. The hair is brittle, and hair loss occurs. Heart rate and temperature are lowered. The patient may complain of lethargy, fatigue, and intolerance to cold. Mentation may be impaired. Thyroid enlargement may occur if reduced levels of T3 and T4 promote excessive release of TSH.
Causes
Hypothyroidism in the adult is usually due to malfunction of the thyroid itself. In iodine-sufficient countries, the principal cause is chronic autoimmune thyroiditis (Hashimoto thyroiditis). Other causes are insufficient iodine in the diet, surgical removal of the thyroid, and destruction of the thyroid by radioactive iodine. Adult hypothyroidism may also result from insufficient secretion of TSH and TRH.
Therapeutic Strategy
Hypothyroidism in adults requires replacement therapy with thyroid hormones. In almost all cases, treatment must continue lifelong. Today, the standard replacement regimen consists of levothyroxine (T4) alone. Combined therapy with levothyroxine plus liothyronine (T3) is an option. However, with only three exceptions, all studies to date indicate that combined T3/T4 offers no advantage over T4 alone. (Remember, when we give T4, much of it is rapidly converted to T3, the active form of the hormone.) When replacement doses of T4 are adequate, they can eliminate all signs and symptoms of thyroid deficiency.
Hypothyroidism During Pregnancy
Maternal hypothyroidism can result in permanent neuropsychological deficits in the child. We have long known that congenital hypothyroidism can cause developmental problems (see next section “Hypothyroidism in Infants”). However, it was not until 1999 that researchers demonstrated that maternal hypothyroidism—in the absence of fetal hypothyroidism—can decrease IQ and other aspects of neuropsychological function in the child. The effect of maternal hypothyroidism is limited largely to the first trimester, a time during which the fetus is unable to produce thyroid hormones of its own. By the second trimester, the fetal thyroid gland is fully functional, and hence the fetus can supply its own hormones from then on. Therefore to help ensure healthy fetal development, maternal hypothyroidism must be diagnosed and treated very early. Unfortunately, symptoms of hypothyroidism are often nonspecific (e.g., irritability, tiredness, poor concentration), or there may be no symptoms at all. Accordingly, some authorities now recommend routine screening for hypothyroidism as soon as pregnancy is confirmed. If hypothyroidism is diagnosed, replacement therapy should begin immediately.
When women taking thyroid supplements become pregnant, dosage requirements usually increase—often by as much as 50%. The need for increased dosage begins between weeks 4 and 8 of gestation, levels off at about week 16, and then remains steady until parturition. To ensure adequate hormone levels, some authorities increase T4 dosage by 30% as soon as pregnancy is confirmed. Further adjustments are based on serum TSH levels, which should be monitored closely.
Hypothyroidism in Infants
Clinical Presentation
Hypothyroidism in newborns may be permanent or transient. In either case, congenital hypothyroidism can cause delay in mental development and derangement of growth. In the absence of thyroid hormones, the child develops a large and protruding tongue, potbelly, and dwarfish stature. Development of the nervous system, bones, teeth, and muscles is impaired.
Causes
Congenital hypothyroidism usually results from a failure in thyroid development. Other causes include autoimmune disease, severe iodine deficiency, TSH deficiency, and exposure to radioactive iodine in utero.
Therapeutic Strategy
Hypothyroidism in newborns requires replacement therapy with thyroid hormones. If treatment is initiated within a few days of birth, physical and mental development will be normal. However, if therapy is delayed beyond 3 to 4 weeks, some permanent disability will be evident, although the physical effects of thyroid deficiency will reverse.
In all children, replacement therapy should continue for 3 years, after which it should be stopped for 4 weeks. The objective is to determine whether thyroid deficiency is permanent or transient. If TSH rises, indicating thyroid hormone production is low, we know the deficiency is permanent, so replacement therapy should resume. If TSH and T4 normalize, we know the deficiency was transient, and hence further replacement therapy is unnecessary.
Hyperthyroidism
There are two major forms of hyperthyroidism: Graves disease and toxic nodular goiter (also known as Plummer disease). Of the two disorders, Graves disease is more common. Signs and symptoms of both disorders are similar. The principal difference is that Graves disease may cause exophthalmos, whereas toxic nodular goiter does not.
Graves Disease
Graves disease is the most common cause of excessive thyroid hormone secretion. This disorder occurs most frequently in women aged 20 to 40 years. The incidence in females is 6 times greater than in males.
Clinical Presentation
Most clinical manifestations result from elevated levels of thyroid hormone. Heartbeat is rapid and strong, and dysrhythmias and angina may develop. The central nervous system is stimulated, resulting in nervousness, insomnia, rapid thought flow, and rapid speech. Skeletal muscles may weaken and atrophy. Metabolic rate is raised, resulting in increased heat production, increased body temperature, intolerance to heat, and skin that is warm and moist. Appetite is increased. However, despite increased food consumption, weight loss occurs if caloric intake fails to match the increase in metabolic rate. Collectively, the above signs and symptoms are referred to as thyrotoxicosis.
In addition to thyrotoxicosis, patients with Graves disease often present with exophthalmos. The underlying cause is an immune-mediated infiltration of the extraocular muscles and orbital fat by lymphocytes, macrophages, plasma cells, mast cells, and mucopolysaccharides.
Cause
Thyroid stimulation in Graves disease is caused by thyroid-stimulating immunoglobulins (TSIs), which are antibodies produced by an autoimmune process. TSIs increase thyroid activity by stimulating receptors for TSH on the thyroid gland. That is, TSIs mimic the effects of TSH on thyroid function. TSIs are not responsible for exophthalmos.
Treatment
Treatment for Graves disease is directed at decreasing the production of thyroid hormones. Three modalities are employed: (1) surgical removal of thyroid tissue, (2) destruction of thyroid tissue with radioactive iodine, and (3) suppression of thyroid hormone synthesis with an antithyroid drug (methimazole or propylthiouracil). Radiation is the preferred treatment for adults, whereas antithyroid drugs are preferred for younger patients.
Beta blockers and nonradioactive iodine may be used as adjunctive therapy. Beta blockers suppress tachycardia by blocking beta receptors on the heart. Nonradioactive iodine inhibits synthesis and release of thyroid hormones.
Because exophthalmos is not the result of hyperthyroidism per se, this condition is not improved by lowering thyroid hormone production. If exophthalmos is severe, it can be treated with surgery or with high doses of oral glucocorticoids.
Toxic Nodular Goiter (Plummer Disease)
Toxic nodular goiter is the result of a thyroid adenoma. Clinical manifestations are much like those of Graves disease, except exophthalmos is absent. Toxic nodular goiter is a persistent condition that rarely undergoes spontaneous remission. Treatment modalities are the same as for Graves disease. However, if an antithyroid drug is used, symptoms return rapidly when the drug is withdrawn. Accordingly, surgery and radiation, which provide long-term control, are often preferred.
Thyrotoxic Crisis (Thyroid Storm)
Thyrotoxic crisis can occur in patients with severe thyrotoxicosis when they undergo major surgery or develop a severe intercurrent illness (e.g., infection, sepsis). The syndrome is characterized by profound hyperthermia (105° F or even higher), severe tachycardia, restlessness, agitation, and tremor. Unconsciousness, coma, hypotension, and heart failure may ensue. These symptoms are produced by excessive levels of thyroid hormones.
Thyrotoxic crisis can be life-threatening and requires immediate treatment. High doses of potassium iodide or strong iodine solution are given to suppress thyroid hormone release. Methimazole is given to suppress thyroid hormone synthesis. A beta blocker is given to reduce heart rate. Additional measures include sedation, cooling, and giving glucocorticoids and intravenous (IV) fluids.
Thyroid Hormone Preparations for Hypothyroidism
Thyroid hormones are available as pure, synthetic compounds and as extracts of animal thyroid glands. All preparations have qualitatively similar effects. The synthetic preparations are more stable and better standardized than the animal gland extracts. As a result, the synthetics are preferred to the natural products. Properties of thyroid hormone preparations are shown in Table 47.2.
TABLE 47.2
Thyroid Hormone Preparations
| Generic Name | Trade Names | Dosage Forms | Approximate Equivalent Dosage* | Description |
| Levothyroxine | Levothroid, Levoxyl, Synthroid | Tablets, injection | 50–60 mcg | Synthetic preparation of T4 identical to the naturally occurring hormone |
| Liothyronine | Cytomel, Triostat | Tablets, injection | 15–37 mcg | Synthetic preparation of T3 identical to the naturally occurring hormone |
| Liotrix | Thyrolar | Tablets | 60 mcg | Synthetic T4 plus synthetic T3 in a 4 : 1 fixed ratio |
| Thyroid | Armour Thyroid, Nature-Throid, Thyroid USP, Westhroid | Tablets, capsules | 60 mg | Desiccated animal thyroid glands (rarely used today) |
