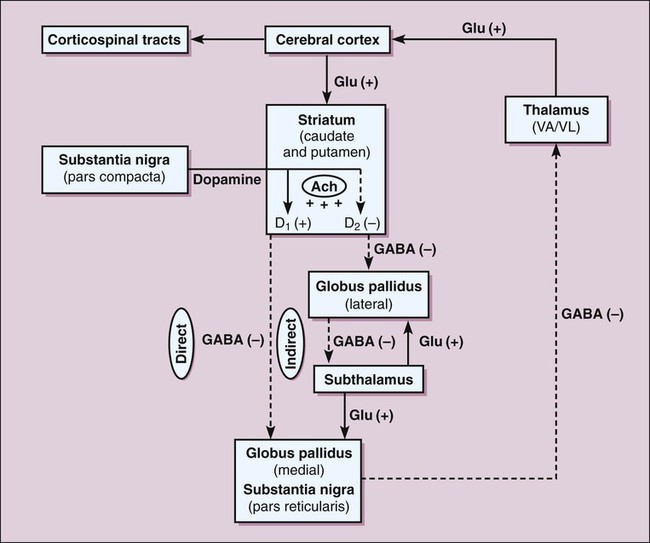
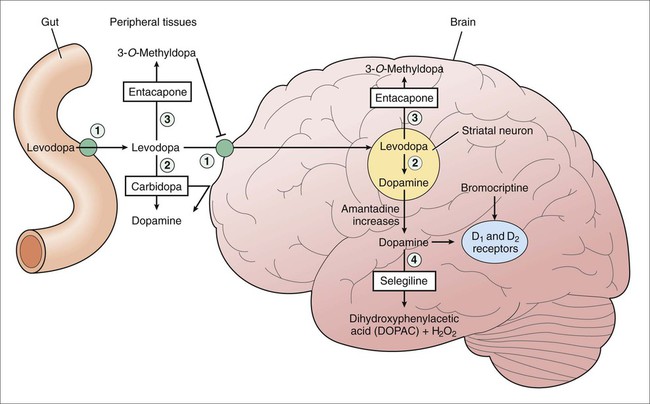
The basal ganglia function via a series of reciprocal innervations among themselves and the cortex (Fig. 24-1). The striatum receives input from the cerebral cortex and substantia nigra and then sends output to the thalamus via the globus pallidus. The thalamus then feeds information back to the motor area of the cortex. Two pathways connect the striatum and the thalamus: a direct pathway, which is excitatory, and an indirect pathway, which is inhibitory. In patients with PD, the degeneration of dopaminergic neurons results in decreased activity in the direct pathway and increased activity in the indirect pathway. As a result, thalamic feedback to the cortex is reduced, and patients exhibit bradykinesia and rigidity. Dopamine levels in the basal ganglia can be increased by various drugs in different ways. Levodopa increases dopamine levels by increasing dopamine synthesis; selegiline by inhibiting dopamine breakdown; and amantadine by increasing dopamine release from neurons. Carbidopa and entacapone increase the amount of levodopa that enters the brain and thereby enhance dopamine synthesis. The sites of action of these drugs are illustrated in Figure 24-2. Levodopa is absorbed from the proximal duodenum by the same process that absorbs large neutral amino acids (see Fig. 24-2). Dietary amino acids compete with levodopa for transport into the circulation, and amino acids can also reduce the transport of levodopa into the brain. For these reasons, the ingestion of high-protein foods can decrease the effectiveness of levodopa, and a protein-restricted diet may improve the response to levodopa in some patients. Levodopa has important interactions with a number of medications. Drugs that delay gastric emptying, such as anticholinergic drugs, can slow levodopa absorption and reduce its peak serum concentration. Drugs that promote gastric emptying (e.g., antacids) can increase levodopa bioavailability. Nonselective monoamine oxidase inhibitors (MAOIs), for example, the antidepressant phenelzine, inhibit the breakdown of dopamine and sometimes cause a hypertensive crisis in patients receiving levodopa. Antipsychotic drugs block dopamine receptors and can reduce the effectiveness of levodopa and exacerbate motor dysfunction. Because clozapine is much less likely to do this than other antipsychotic drugs, it is often used to manage psychotic reactions in patients receiving levodopa (see Chapter 22). Carbidopa, a structural analogue of levodopa, inhibits LAAD thereby reducing the conversion of levodopa to dopamine in peripheral tissues and so increasing the amount of levodopa that enters the brain (see Fig. 24-2). Carbidopa is highly ionized at physiologic pH, and it does not cross the blood-brain barrier. For this reason, it does not inhibit the formation of dopamine in the CNS. Selegiline inhibits monoamine oxidase type B (MAO-B) and thereby prevents the oxidation of dopamine to dihydroxyphenylacetic acid and hydrogen peroxide, as shown in Figure 24-2. By this action, selegiline increases dopamine levels in the basal ganglia and decreases the formation of hydrogen peroxide. In the presence of iron, hydrogen peroxide is converted to hydroxyl and hydroxide free radicals that may participate in the degeneration of nigrostriatal neurons in patients with PD. Adverse effects are listed in Table 24-1. Unlike the nonselective MAOIs used in treating mood disorders, selegiline does not inhibit monoamine oxidase type A (MAO-A), an enzyme that catalyzes the degradation of catecholamines. For this reason, selegiline is much less likely to cause hypertension when it is administered in combination with sympathomimetic amines or when it is taken with foods that contain tyramine. The drug’s selectivity for MAO-B, however, is lost when it is given in higher doses, so the potential for food interaction still exists. Selegiline can cause adverse effects if it is administered with meperidine or with selective serotonin reuptake inhibitors (SSRIs; e.g., fluoxetine), as noted in Chapter 22. TABLE 24-1 Major Adverse Effects and Major Interactions of Selected Drugs for Neurodegenerative Diseases
Drugs for Neurodegenerative Diseases
Parkinson Disease
Etiology and Pathogenesis
Drugs That Increase Dopamine Levels
Levodopa
Pharmacokinetics
Interactions
Carbidopa
Selegiline
Mechanisms and Pharmacologic Effects
Adverse Effects and Interactions
DRUG
MAJOR ADVERSE EFFECTS
MAJOR DRUG INTERACTIONS
Drugs for Parkinson Disease
Drugs That Increase Dopamine Levels
Amantadine
Dry mouth, hypotension, livedo reticularis, nausea, restlessness, sedation, and vivid dreams
Benztropine and trihexyphenidyl potentiate CNS side effects.
Levodopa-carbidopa
Agitation; arrhythmias; delirium; distorted thinking, hallucinations, and other psychotic effects; dyskinesias; hypotension; nausea and vomiting; nightmares or vivid dreams; and sedation
Antacids may increase bioavailability. Anticholinergic drugs may reduce peak serum level. Antipsychotic drugs, such as haloperidol, may decrease effects. Nonselective MAOIs, such as phenelzine, may cause a hypertensive crisis.
Selegiline
Confusion; dyskinesias; hallucinations; hypotension; insomnia; and nausea
Severe reactions may result if taken with meperidine or with fluoxetine or other SSRIs.
Rasagiline
Same as selegiline
Same as selegiline.
Tolcapone
Diarrhea and nausea
Unknown.
Entacapone
Diarrhea and nausea
Unknown.
Dopamine Receptor Agonists
Bromocriptine
Confusion, decreased prolactin levels, dry mouth, dyskinesias, hallucinations, nausea, orthostatic hypotension, sedation, and vivid dreams
Dopamine antagonists may reduce effects.
Pramipexole
Dizziness, hallucinations, insomnia, nausea and vomiting, and sedation
Cimetidine inhibits renal excretion and increases serum levels.
Ropinirole
Same as pramipexole
Ciprofloxacin increases serum levels.
Rotigotine
Somnolence, slight BP and HR increase, site irritation
Sulfite sensitivity; no major drug interactions.
Acetylcholine Receptor Antagonists
Benztropine
Agitation, confusion, constipation, delirium, dry mouth, memory loss, urinary retention, and tachycardia
Additive anticholinergic effect with antihistamines and phenothiazines.
Trihexyphenidyl
Same as benztropine
Same as benztropine.
Drugs for Huntington Disease
Diazepam
Arrhythmias, CNS depression, drug dependence, hypotension, and mild respiratory depression
Alcohol and other CNS depressants potentiate effects. Cimetidine increases and rifampin decreases serum levels.
Haloperidol
Extrapyramidal side effects and increased prolactin levels
Barbiturates and carbamazepine decrease and quinidine increases serum levels.
Drugs for Alzheimer Disease
Donepezil
Bradycardia, diarrhea, gastrointestinal bleeding, and nausea and vomiting
Anticholinergic drugs inhibit effects.
Rivastigmine
Risk of bradycardia and AV block; nausea and vomiting, anorexia, weight loss
Anticholinergic drugs inhibit effects. Nicotine use increases oral clearance.
Galantamine
Risk of bradycardia and AV block; nausea and vomiting
Anticholinergic drugs inhibit effects. Inhibitors of CYP2D6 increase serum levels.
Memantine
Confusion, dizziness, drowsiness, headache, insomnia
Carbonic anhydrase inhibitors reduce renal elimination of memantine.
Drugs for Multiple Sclerosis
Baclofen
Dizziness, fatigue, and weakness
Unknown.
Interferon beta-1b
Chills, diarrhea, fever, headache, hypertension, myalgia, pain, and vomiting
Increases serum levels of zidovudine.
Prednisone
Aggravation of diabetes mellitus, gastrointestinal bleeding, mood changes, pancreatitis, and seizures
Barbiturates, carbamazepine, phenytoin, and rifampin decrease serum levels.
Drugs for Amyotrophic Lateral Sclerosis
Baclofen
Dizziness, fatigue, and weakness
Unknown.
Gabapentin
Ataxia, dizziness, drowsiness, nystagmus, and tremor
Antacids decrease serum levels.
Riluzole
Asthenia, diarrhea, dizziness, drowsiness, increased hepatic enzyme levels, nausea and vomiting, paresthesias, and vertigo
Quinolones and theophylline can increase serum levels. Omeprazole, rifampin, and smoking can decrease serum levels. ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree