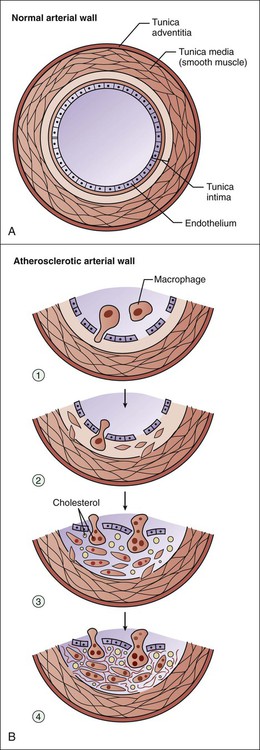
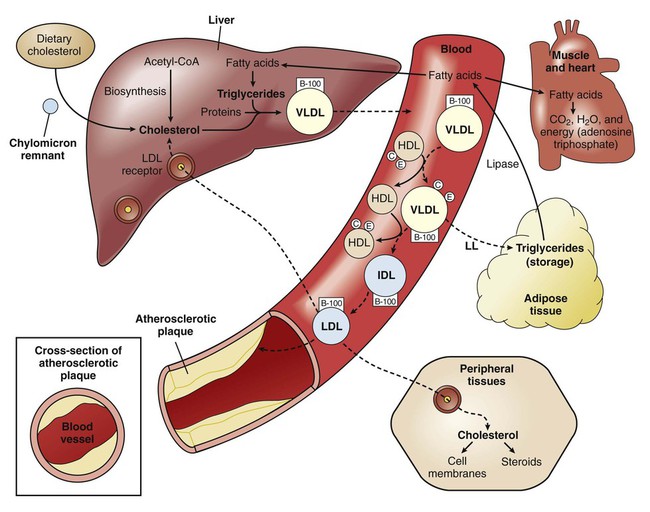
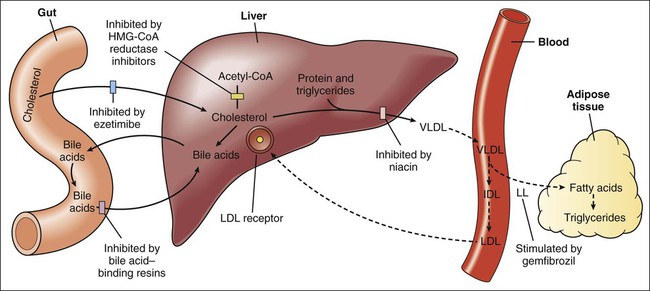
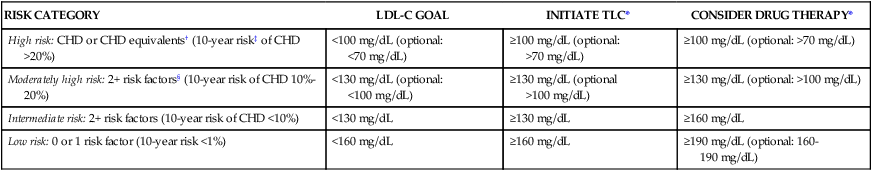
Whereas hyperlipidemia and hyperlipoproteinemia are general terms for elevated concentrations of lipids and lipoproteins in the blood, hypercholesterolemia and hypertriglyceridemia refer specifically to high concentrations of cholesterol and triglycerides, respectively. Hypercholesterolemia contributes to the pathogenesis of atherosclerosis and has been causally associated with coronary artery disease and other atherosclerotic vascular diseases (Fig. 15-1). Hypertriglyceridemia is a risk factor for pancreatitis, but its role in the development of atherosclerosis and heart disease remains uncertain. Clinical trials of drugs that reduce serum triglycerides have not consistently reduced cardiovascular events and have not reduced mortality. Because lipids are insoluble in plasma water, they are transported in the blood in the form of lipoproteins. These substances have a core of hydrophobic (water-evading) lipids surrounded by a shell of hydrophilic (water-attracting) proteins and portions of phospholipids. There are numerous types of lipoproteins, including chylomicrons, very-low-density lipoproteins (VLDLs), low-density lipoproteins (LDLs), intermediate-density lipoproteins, HDLs, and lipoprotein (a). The various types are distinguished in terms of their buoyant density, lipid and protein composition, and role in lipid transport. Moreover, each type is associated with a unique group of apoproteins. Some of the apoproteins are exchanged between different types of lipoproteins as they transport lipids to various tissues. The composition and metabolism of lipoproteins is depicted in Box 15-1. Chylomicrons are involved primarily in the transport of dietary lipids from the gut to the adipose tissue and liver. When cholesterol and triglycerides are ingested, they are emulsified in the intestines by the bile acids and other bile secretions, and the emulsified lipids are combined with proteins to form chylomicrons in the gut wall. After chylomicrons are secreted into the circulation, they deliver triglycerides to adipose tissue via the action of a lipoprotein lipase located in the vascular endothelial cells. By this process, chylomicrons are converted to a cholesterol-rich chylomicron remnant, which transports cholesterol to the liver. Golgi bodies in the liver form VLDLs from triglycerides, cholesterol, and protein and then secrete the VLDLs into the circulation. The VLDLs deliver triglycerides to adipose tissue in the same manner as do the chylomicrons. During the process, the VLDLs are transformed into intermediate-density lipoproteins and LDLs that contain a high percentage of cholesterol (Fig. 15-2). The LDLs can also deliver cholesterol to nascent atheromas and thereby contribute to the development of atherosclerosis (see Fig. 15-1). In atheromas, cholesterol is phagocytosed by macrophages, which are transformed into foam cells as they become filled with oxidized cholesterol. Hyperlipoproteinemia occurs as a result of genetic or environmental factors that increase the formation of lipoproteins or reduce the clearance of lipoproteins from the circulation. These factors include biochemical defects in lipoprotein metabolism, excessive dietary intake of lipids, endocrine abnormalities, and use of drugs that perturb lipoprotein formation or catabolism. Table 15-1 provides information about the characteristics and types of hyperlipoproteinemia. TABLE 15-1 Types and Characteristics of Hyperlipoproteinemia *Use of β-adrenoceptor antagonist may decrease the high-density–lipoprotein (HDL) cholesterol concentration. †Use of thiazide diuretics may increase the total cholesterol concentration. The NCEP guidelines (Table 15-2) establish LDL-C goals and levels for initiating therapeutic lifestyle changes (TLCs) and drug therapy for persons in different risk categories. For high-risk patients (who already have CHD or have CHD risk equivalents), the basic goal is to achieve an LDL-C level of less than 100 mg/dL, and TLC and drug therapy should be initiated if the patient’s LDL-C level is higher than 100 mg/dL. The updated guidelines suggest an optional LDL-C goal for high-risk patients of less than 70 mg/dL, particularly for those whose LDL-C level is less than 100 mg/dL at baseline. This optional goal is based on clinical trials that show that high-risk patients benefit from LDL-C reduction regardless of their baseline level. TABLE 15-2 National Cholesterol Education Program Guidelines for Management of High Blood Cholesterol Levels for Adults in Different Risk Categories* *LDL-C levels at which TLC or drug therapy initiated. †Includes myocardial infarction, angina, myocardial ischemia, noncoronary forms of atherosclerosis, and diabetes mellitus. ‡Electronic 10-year risk calculators available at www.nhlbi.nih.gov/guidelines/cholesterol. §Risk factors include cigarette smoking, hypertension, low HDL-C, family history of premature CHD, and age (see chapter text for details). From Adult Treatment Panel III Guidelines, issued in 2001 and updated in 2004.
Drugs for Hyperlipidemia
Overview
Lipoproteins and Lipid Transport
Chylomicrons
Very-Low-Density and Low-Density Lipoproteins
Causes and Types of Hyperlipoproteinemia
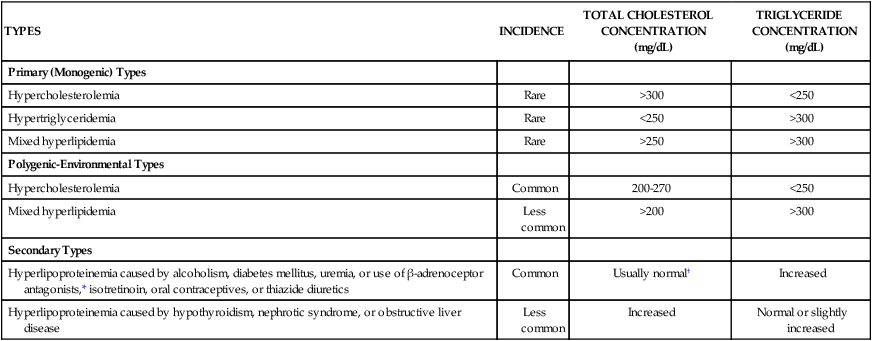
TYPES
INCIDENCE
TOTAL CHOLESTEROL CONCENTRATION (mg/dL)
TRIGLYCERIDE CONCENTRATION (mg/dL)
Primary (Monogenic) Types
Hypercholesterolemia
Rare
>300
<250
Hypertriglyceridemia
Rare
<250
>300
Mixed hyperlipidemia
Rare
>250
>300
Polygenic-Environmental Types
Hypercholesterolemia
Common
200-270
<250
Mixed hyperlipidemia
Less common
>200
>300
Secondary Types
Hyperlipoproteinemia caused by alcoholism, diabetes mellitus, uremia, or use of β-adrenoceptor antagonists,* isotretinoin, oral contraceptives, or thiazide diuretics
Common
Usually normal†
Increased
Hyperlipoproteinemia caused by hypothyroidism, nephrotic syndrome, or obstructive liver disease
Less common
Increased
Normal or slightly increased
Guidelines for Management of Hypercholesterolemia
RISK CATEGORY
LDL-C GOAL
INITIATE TLC*
CONSIDER DRUG THERAPY*
High risk: CHD or CHD equivalents† (10-year risk‡ of CHD >20%)
<100 mg/dL (optional: <70 mg/dL)
≥100 mg/dL (optional: >70 mg/dL)
≥100 mg/dL (optional: >70 mg/dL)
Moderately high risk: 2+ risk factors§ (10-year risk of CHD 10%-20%)
<130 mg/dL (optional: <100 mg/dL)
≥130 mg/dL (optional >100 mg/dL)
≥130 mg/dL (optional: >100 mg/dL)
Intermediate risk: 2+ risk factors (10-year risk of CHD <10%)
<130 mg/dL
≥130 mg/dL
≥160 mg/dL
Low risk: 0 or 1 risk factor (10-year risk <1%)
<160 mg/dL
≥160 mg/dL
≥190 mg/dL (optional: 160-190 mg/dL)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs for Hyperlipidemia
Only gold members can continue reading. Log In or Register to continue