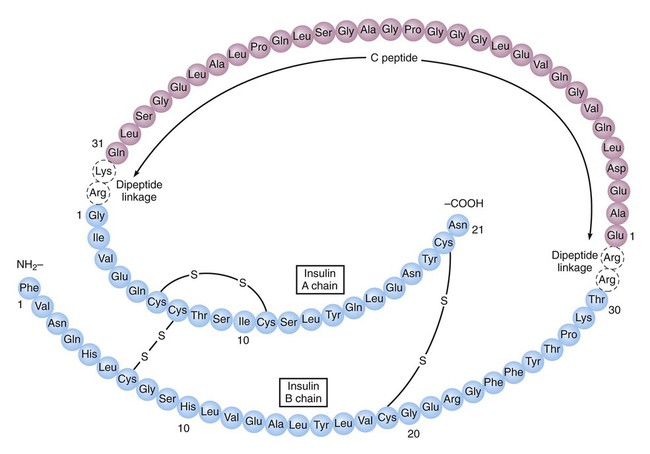
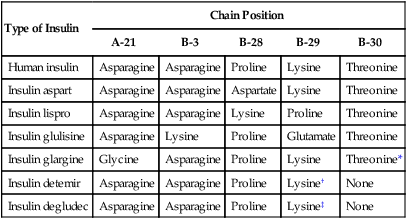
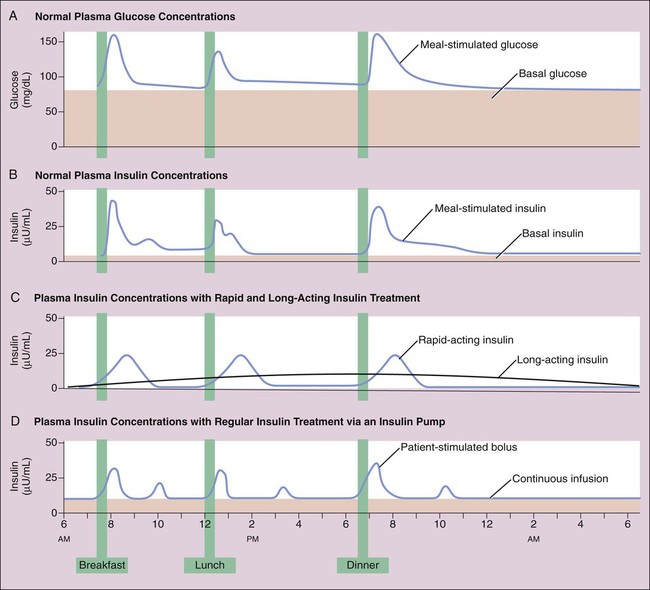
As shown in Box 35-1, proinsulin is converted to insulin and C peptide. Insulin consists of two peptide chains (the A chain and the B chain), which are linked by two disulfide (-S-S-) bridges. Although both insulin and C peptide are released in response to rising glucose concentrations, the physiologic role of C peptide remains unknown. Insulin secretion has meal-stimulated and basal components. Insulin release is activated by the rise in blood glucose concentration that follows the digestion and absorption of carbohydrates (Fig. 35-1A and 35-1B). Insulin is released at the rate of 1 unit (U) per 10 g of dietary carbohydrate, and its level usually peaks within 1 hour of eating. Insulin promotes the uptake and storage of glucose and other ingested nutrients, and the postprandial (postmeal) plasma concentrations of both insulin and glucose return to preprandial (premeal) levels within 2 hours. Basal secretion, which usually ranges from 0.5 to 1 U of insulin per hour, serves to retard hepatic glucose output during the postabsorptive state. Type 2 diabetes (Box 35-2) is a heterogeneous disease that usually has its onset after the patient reaches 30 years of age and is often associated with a significant degree of insulin resistance and obesity. Insulin resistance can be caused by the presence of insulin antibodies or by defects in insulin receptors and signal transduction mechanisms in target organs. Patients with type 2 diabetes are less susceptible to developing ketonemia and ketoacidosis than are type 1 patients. Most patients with type 2 diabetes have normal or elevated concentrations of insulin and do not require exogenous insulin for survival. Type 2 diabetes is usually treated with oral antidiabetic medications in combination with dietary modifications and exercise, but some patients benefit from insulin treatment. Based on their onset and duration of action, insulin preparations are classified as short-acting, rapid-acting, intermediate-acting, and long-acting (Table 35-1). TABLE 35-1
Drugs for Diabetes Mellitus
Overview
Pancreatic Hormones
Insulin
Secretion
Diabetes Mellitus
Classification
Insulin Preparations
Administration and Absorption
TYPE OF INSULIN
ONSET
PEAK
DURATION
Rapid-Acting Insulin
Insulin aspart
10-20 min
40-50 min
3-5 hr
Insulin lispro
15-30 min
30-90 min
3-5 hr
Insulin glulisine
20-30 min
30-90 min
1-2.5 hr
Short-Acting Insulin
Regular insulin
30-60 min
2-5 hr
5-8 hr
Intermediate-Acting Insulin
Isophane (NPH) insulin
1-2 hr
4-12 hr
18-24 hr
Long-Acting Insulin
Insulin glargine
1-1.5 hr
None
20-24 hr
Insulin detemir
1-2 hr
6-8 hr
Up to 24 hr
Insulin degludec
1-2 hr
None
>24 hr
Inhaled Insulin
Human insulin liquid
5-10 min
1 hr
5-10 hr ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs for Diabetes Mellitus
Only gold members can continue reading. Log In or Register to continue