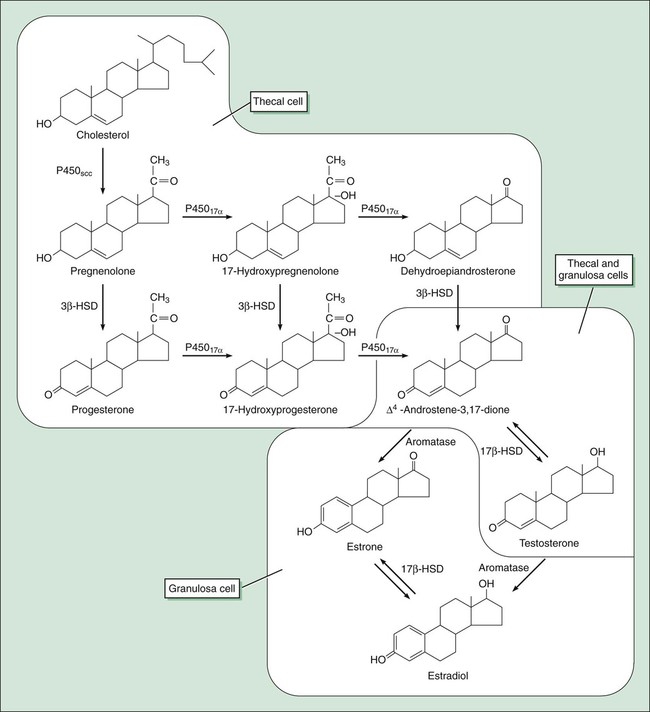
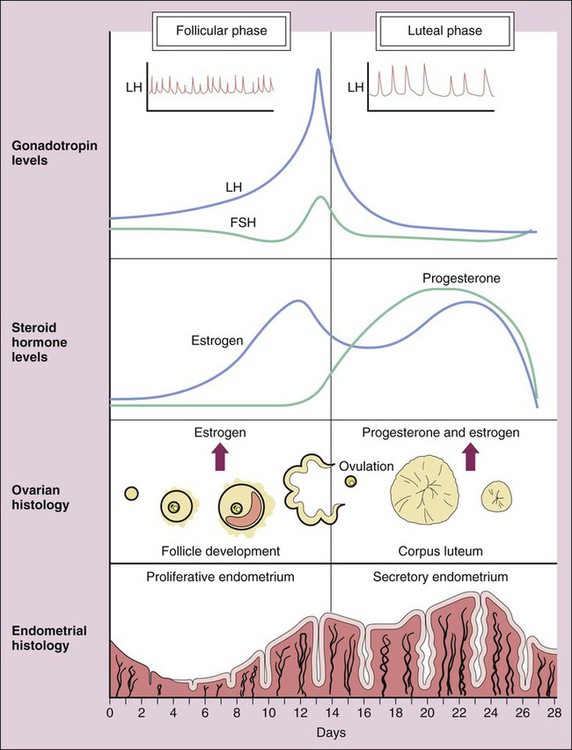
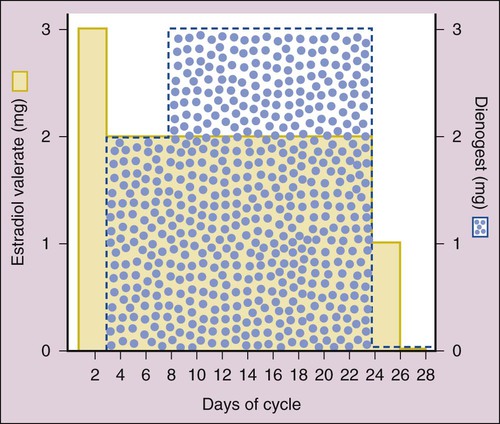
Human reproduction involves a cascade of hormonal secretions, beginning with the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus. As described in Chapter 31, GnRH stimulates the pituitary to release two gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These gonadotropins then stimulate the production of steroids and gametes by the ovary in the female and by the testis in the male. As shown in Figure 34-1, pregnenolone is the precursor to progesterone. It is also the precursor to dehydroepiandrosterone and androstenedione (two androgens secreted by the adrenal gland and discussed in Chapter 33) and to testosterone (the major androgen in males). The adrenal and gonadal androgens are converted to estrogens by aromatase, an enzyme that forms the aromatic A-ring necessary for the selective high-affinity binding of estradiol, estrone, and estriol to estrogen receptors. The pattern of hormonal changes occurring during the menstrual cycle is depicted in Figure 34-2. During the follicular phase of the cycle, ovarian follicles are recruited and a dominant estrogen-secreting follicle develops. Estrogen levels gradually increase, whereas progesterone levels remain very low. A surge of LH is released at mid-cycle in response to positive estrogen feedback to the pituitary gland, and this LH surge triggers ovulation. During the luteal phase of the cycle, the follicle becomes the corpus luteum (“yellow body”) that secretes both estrogen and progesterone in response to LH. Together, these hormones prepare the uterus for implantation of a fertilized egg as the endometrium becomes more vascular and secretory. If pregnancy does not occur, the corpus luteum ceases to produce estrogen and progesterone, resulting in menstruation. If pregnancy occurs, the placenta produces human chorionic gonadotropin, which maintains the production of progesterone by the corpus luteum. After about 3 months, the placenta becomes the predominant source of progesterone. This hormone serves to maintain pregnancy and prevents endometrial sloughing and miscarriage. The natural estrogens include estradiol and conjugated estrogens. Estradiol is an 18-carbon steroid with an aromatic A-ring (see Fig. 34-1). Conjugated equine estrogens are sulfate esters of estrone and equilin and can be obtained from the urine of pregnant mares. Ethinyl estradiol and mestranol are synthetic derivatives of estradiol. Several long-acting formulations of estradiol are available for transdermal or intramuscular administration. Transdermal estradiol systems slowly release the drug for absorption through the skin. These preparations are formulated for twice-weekly or weekly application. Estradiol cypionate and estradiol valerate are long-acting esters of estradiol that are slowly absorbed after intramuscular administration and provide effective plasma concentrations of estradiol for several weeks. Estradiol valerate is also contained in a variable dose oral contraceptive (NATAZIA), in which the estrogen dose is stepped up and the progestin dose is stepped down during each menstrual cycle (Fig. 34-3). Studies consistently show that estrogens relieve symptoms of menopause in up to 90% of women. These symptoms include hot flashes or flushes that consist of alternating chills and sweating accompanied by nausea, dizziness, headache, tachycardia, and palpitations (Box 34-1). Episodes of these symptoms often occur several times a day, but night sweats are particularly common. These symptoms occur in association with surges in GnRH and gonadotropins that result from the lack of estrogen feedback inhibition. The gonadotropin surges alter hypothalamic thermoregulatory centers, leading to the symptoms described earlier in this paragraph.
Drugs Affecting Fertility and Reproduction
Overview
Biosynthesis of Gonadal Steroids
Physiologic Actions of Estrogens and Progesterone
Estrogens and Progestins
Estrogens
Drug Properties
Chemistry.
Preparations.
Hormone Replacement Therapy
Therapeutic Effects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs Affecting Fertility and Reproduction
Only gold members can continue reading. Log In or Register to continue