Joint destruction is caused by an autoimmune process in which the immune system mounts an attack against synovial tissue. During the attack, mast cells, macrophages, and T lymphocytes produce cytokines and cytotoxins—compounds that promote inflammation and joint destruction. The cytokines of greatest importance are tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6, interferon gamma, platelet-derived growth factor, and granulocyte-macrophage colony-stimulating factor. Why the immune system attacks joints is unclear.
Overview of Therapy
Treatment is directed at (1) relieving symptoms (pain, inflammation, and stiffness), (2) maintaining joint function and range of motion, (3) minimizing systemic involvement, and (4) delaying disease progression. To achieve these goals, a combination of pharmacologic and nonpharmacologic measures is used.
Nondrug Measures
Nondrug measures for managing RA include physical therapy, exercise, and surgery. Physical therapy may consist of massage, warm baths, and applying heat to the affected regions. These procedures can enhance mobility and reduce inflammation. A balanced program of rest and exercise can decrease joint stiffness and improve function. However, excessive rest and excessive exercise should be avoided: too much rest will foster stiffness, and too much activity can intensify inflammation.
Orthopedic surgery has made marked advances. For patients with severe disease of the hip or knee, total joint replacement can be performed. When joints of the hands or wrists have been damaged severely, function can be improved through removal of the diseased synovium and repair of ruptured tendons. Plastic implants can help correct deformities.
A complete program of treatment should include patient education and counseling. The patient should be informed about the nature of RA, the possible consequences of joint degeneration, management measures, and the benefits and limitations of drug therapy. If loss of mobility limits function at home, on the job, or in school, consultation with a social worker, occupational therapist, or specialist in vocational rehabilitation may be appropriate.
Drug Therapy
Antiarthritic drugs can produce symptomatic relief, and some drugs, if started very early in the disease process, can induce protracted remission. However, remission is rarely complete, and the disease typically advances steadily. As a result, drug therapy is chronic and hence success requires patient motivation and cooperation.
Classes of Antiarthritic Drugs
The antirheumatic drugs fall into three major groups:
These major groups differ with respect to time course of effects, toxicity, and ability to slow RA progression.
The NSAIDs provide rapid relief of symptoms but do not prevent joint damage and do not slow disease progression. The NSAIDs are safer than DMARDs and glucocorticoids; thus treatment with NSAIDs requires less vigorous monitoring.
Like the NSAIDs, glucocorticoids provide rapid relief of symptoms. In addition, they can slow disease progression. Unfortunately, although glucocorticoids are effective, with long-term use they can cause serious toxicity. As a result, treatment is usually limited to short courses.
By definition, DMARDs are drugs that reduce joint destruction and slow disease progression. However, benefits develop more slowly than with the NSAIDs. The DMARDs are more toxic than NSAIDs, and therefore close monitoring is required. In the discussion that follows, DMARDs are subdivided into two basic groups—nonbiologic DMARDs (traditional DMARDs) and biologic DMARDs—based on their molecular size and method of production. The nonbiologic DMARDs are small molecules that are synthesized using conventional chemical techniques. In contrast, the biologic DMARDs are large molecules that are produced through recombinant DNA technology.
Drug Selection
Management of RA is overseen by a rheumatologist or other specialists. DMARDs, in particular, are associated with significant effects and sometimes dangerous risks. Lifespan considerations are a special concern. (See Box 57.1.)
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Disease-Modifying Antirheumatic Drugs
| Life Stage | Considerations or Concerns |
| Children | Biologic DMARDs: Children and adolescents taking TNF antagonists have developed lymphoma and other malignancies. |
| Pregnant women | Biologic DMARDs: TNF antagonists are FDA Pregnancy Risk Category B. Rituximab and abatacept are Pregnancy Risk Category C. Nonbiologic DMARDs: Azathioprine is teratogenic. Both leflunomide and methotrexate can cause fetal death and congenital abnormalities. Hydroxychloroquine may cause fetal ocular toxicity; however, in some conditions such as maternal lupus or malaria, the drug decreases fetal risk associated with the conditions it treats. Sulfasalazine is Pregnancy Risk Category B. |
| Breastfeeding women | Breastfeeding is not recommended for mothers taking DMARDs. |
| Older adults | Elderly patients may be at a greater risk for infection secondary to DMARD immunosuppressive effects. |
Management of RA is aggressive. Current guidelines recommend starting a DMARD early—within 3 months of RA diagnosis for most patients. (See Fig. 57.2.) The aim is to delay joint degeneration. Recall that NSAIDs only provide symptomatic relief; they do not slow disease progression. In contrast, DMARDs may be able to arrest the disease process. By instituting DMARD therapy early—rather than waiting until joint degeneration has advanced to the point at which NSAIDs can no longer control symptoms—it is possible to delay or even prevent serious joint injury.
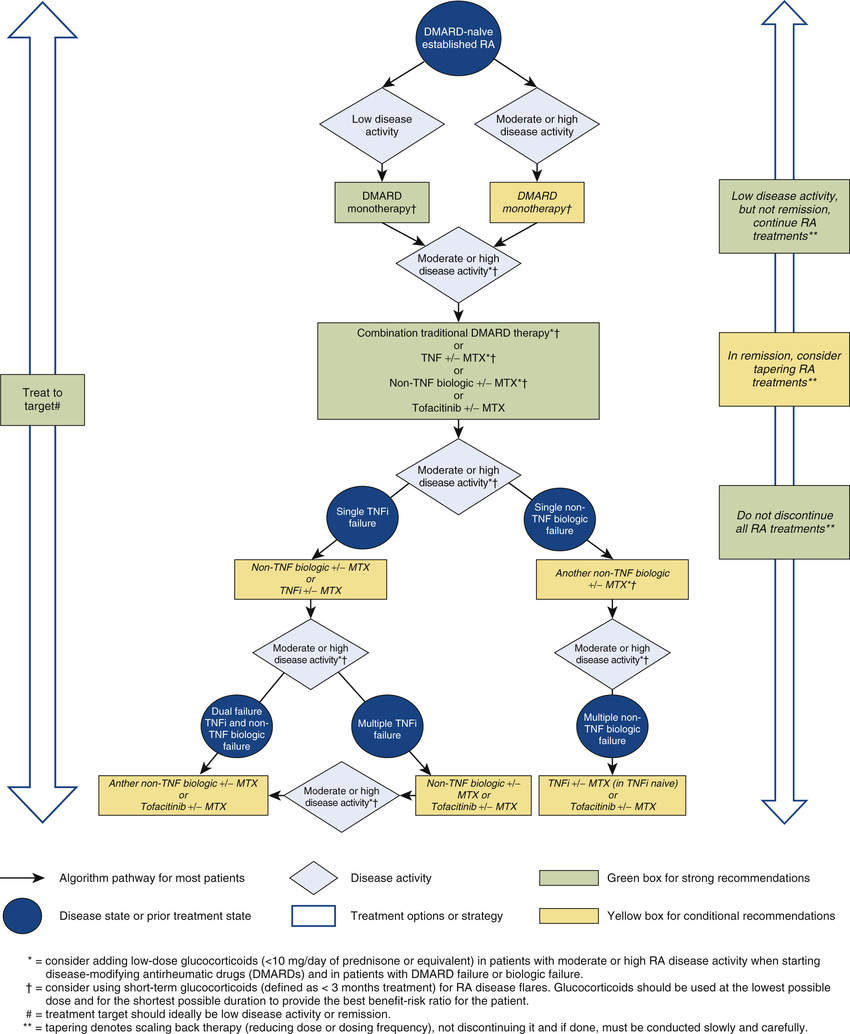
Because the effects of DMARDs take weeks or months to develop, whereas the effects of NSAIDs are immediate, an NSAID is given until the DMARD has had time to act, after which the NSAID can be withdrawn. As in the past, glucocorticoids are generally reserved for short-course management of symptom flare-ups and to control symptoms until DMARDs take effect. If joint injury progresses despite treatment with an initial DMARD (typically methotrexate), another DMARD can be added or substituted.
You can find detailed information on pharmacologic management of RA in clinical guidelines sponsored by the American College of Rheumatology (ACR). The document, 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis, is available at http://www.rheumatology.org/Portals/0/Files/ACR%202015%20RA%20Guideline.pdf.
Nonsteroidal Antiinflammatory Drugs
The basic pharmacology of the NSAIDs is discussed in Chapter 55. Consideration here is limited to their role in RA.
Therapeutic Role
NSAIDs are prescribed for their antiinflammatory and analgesic actions. Both actions result from inhibiting cyclooxygenase (COX). NSAIDs only provide symptomatic relief; they do not slow disease progression. Accordingly, they are usually combined with a DMARD.
Nonsteroidal Antiinflammatory Drug Classification
There are two main classes of NSAIDs: (1) first-generation NSAIDs, which inhibit COX-1 and COX-2; and (2) second-generation NSAIDs (coxibs), which selectively inhibit COX-2. Antiinflammatory and analgesic effects result from inhibiting COX-2, whereas major adverse effects—especially gastroduodenal ulceration—result from inhibiting COX-1. Therefore the selectivity of this drug class results in less gastrointestinal (GI) ulceration than the first-generation NSAIDs, while producing equal therapeutic effects.
Drug Selection
Selection of an NSAID is based largely on efficacy, safety, and cost.
Efficacy
All of the NSAIDs have essentially equal antirheumatic effects. However, individual patients may respond better to one NSAID than to another. Accordingly, it may be necessary to try more than one agent to achieve an optimal response.
Safety and Cost
All prescription-strength NSAIDs carry a boxed warning regarding risk for thrombotic events and GI ulceration and bleeding. Although the risk for GI problems is lessened with COX-2 inhibitors, the risk for thrombotic events may be increased because coxibs inhibit COX-1 to a far lesser degree than traditional NSAIDs. (Recall that COX-1 has a role in the production of thromboxane A2, which participates in blood clotting.) Selection must balance these factors. Coxibs are more expensive than traditional NSAIDs. If symptoms are controlled with a first-generation NSAID and the drug is well tolerated, cost considerations will dictate using that drug. However, if a first-generation NSAID produces serious gastric ulceration and the patient is at low risk for thrombosis, then switching to celecoxib might be appropriate—despite the increased cost.
Dosage
Dosages employed for antiinflammatory effects are considerably higher than those required for analgesia or fever reduction. For example, treatment of RA may require 5.2 g (16 standard tablets) of aspirin a day, compared with only 2.6 g for aches, pain, and fever. Dosages for RA are shown in Table 57.1.
TABLE 57.1
Nonsteroidal Antiinflammatory Drugs: Oral Dosage for Rheumatoid Arthritis
| Generic Name | Trade Name | Daily Dosage |
| FIRST-GENERATION NSAIDS | ||
| Salicylates | ||
| Aspirin | Multiple trade names | 3.6–5.4 g/day in divided doses |
| Magnesium salicylate | Doan’s Extra Strength | 650–1160 mg every 6 hours as needed |
| Salsalate | Generic only | 3 g/day (in 2 or 3 doses) |
| Nonsalicylates | ||
| Diclofenac (salt) | Cambia, Cataflam, Voltaren, Zipsor | Immediate release: 150–200 mg/day (in 3 or 4 doses) Delayed release: 150–200 mg daily (in 2–4 divided doses) Extended release: 100 mg daily or 200 mg daily (in 2 divided doses) |
| Diclofenac (free acid) | Zorvolex | 18–35 mg 3 times a day |
| Diclofenac/misoprostol | Arthrotec | 50 mg diclofenac/200 mcg misoprostol 3 or 4 times daily |
| Diflunisal | Generic only | 250–500 mg twice daily |
| Etodolac | Generic only | Immediate release: 400 mg 2 times/day or 300 mg 2–3 times/day or 500 mg 2 times/day Extended release: 400–1000 mg once daily |
| Fenoprofen | Nalfon | 300–600 mg 3 or 4 times/day |
| Flurbiprofen | Generic only | 200–300 mg/day (in 2–4 doses) |
| Ibuprofen | Motrin, Advil, others | 400–800 mg 3 or 4 times/day |
| Indomethacin | Indocin | 25–50 mg 3 times/day |
| Ketoprofen | Generic only | Immediate release: 150–300 mg/day (in 3 or 4 doses) |
| Extended release: 100–200 mg once a day | ||
| Meclofenamate | Generic only | 200–400 mg/day (in 3 or 4 doses) |
| Meloxicam | Mobic, Mobicox  | 7.5 mg once a day |
| Nabumetone | Generic only | 1–2 g/day (in 1 or 2 doses) |
| Naproxen | Naprosyn | Immediate release: 250–500 mg twice daily |
| Naprelan | Extended release: 750–1500 mg/daily | |
| EC-Naprosyn | Delayed release: 375–500 mg twice daily | |
| Naproxen sodium | Aleve, others | 250–500 mg twice daily |
| Naproxen/esomeprazole | Vimovo | 375–500 mg (naproxen) twice daily |
| Oxaprozin | Daypro | 1.2 g once a day |
| Piroxicam | Feldene | 10 mg twice daily or 20 mg once a day |
| Sulindac | Clinoril | 150–200 mg twice daily |
| Tolmetin | Generic only | 200–600 mg 3 times/day |
| SECOND-GENERATION NSAIDS (COX-2 INHIBITORS) | ||
| Celecoxib | Celebrex | 100–200 mg twice daily |
Glucocorticoids
The glucocorticoids are powerful antiinflammatory drugs that can relieve symptoms of severe RA and may also delay disease progression. For patients with generalized symptoms, oral glucocorticoids are indicated. However, if only one or two joints are affected, intraarticular injections may be employed. Because long-term oral therapy can cause serious toxicity (e.g., osteoporosis, gastric ulceration, adrenal suppression), short-term therapy should be used whenever possible. Most often, glucocorticoids are used for temporary relief until drugs with more slowly developing effects (e.g., methotrexate) can provide control. They may also be employed for flares, which are disease exacerbations that occur in patients whose condition was previously controlled. Long-term therapy should be limited to patients who have failed to respond adequately to all other options. The pharmacology of the glucocorticoids is discussed in Chapter 56.
Nonbiologic (Traditional) Disease-Antimodifying Rheumatic Drugs
As noted, the nonbiologic DMARDs are small molecules produced using conventional synthetic procedures. With several of these drugs, benefits result from immunosuppression. Unlike the NSAIDs, whose benefits are limited to symptomatic relief, the nonbiologic DMARDs can slow disease progression. These drugs are potentially more harmful than the NSAIDs, and clinical responses develop more slowly. The nonbiologic DMARDs cost much less than the biologic DMARDS, largely because the nonbiologic agents are easier to make.
Methotrexate
Methotrexate [Rheumatrex, Trexall] acts faster than all other DMARDs. Therapeutic effects may develop in 3 to 6 weeks. At least 80% of patients improve with this drug. Benefits are the result of immunosuppression secondary to reducing the activity of B and T lymphocytes. Many rheumatologists consider methotrexate the DMARD of first choice, owing to its efficacy, relative safety, low cost, and extensive use in RA. Major toxicities are hepatic fibrosis, bone marrow suppression, GI ulceration, and pneumonitis. Periodic tests of liver and kidney function are mandatory, as are complete blood cell and platelet counts. Methotrexate can cause fetal death and congenital abnormalities and therefore is contraindicated during pregnancy. Recent data suggest that patients using methotrexate for RA may have a reduced life expectancy, owing to increased deaths from cardiovascular disease, infection, and certain cancers (melanoma, lung cancer, and non-Hodgkin lymphoma). For treatment of RA, methotrexate is administered once a week, either orally or by injection. Dosing with folic acid (at least 5 mg/week) is recommended to reduce GI and hepatic toxicity.
Sulfasalazine
Sulfasalazine [Azulfidine, Azulfidine EN-tabs] has been used for decades to treat inflammatory bowel disease. Benefits for RA may result from antiinflammatory and immunomodulatory actions. In patients with RA, sulfasalazine can slow the progression of joint deterioration, sometimes with just 1 month of treatment. Gastrointestinal reactions (nausea, vomiting, diarrhea, anorexia, abdominal pain) are the most common reasons for stopping treatment. These reactions can be minimized by using an enteric-coated formulation and by dividing the daily dosage. Dermatologic reactions (pruritus, rash, urticaria) are also common. Fortunately, serious adverse effects—hepatitis and bone marrow suppression—are rare. To ensure early detection, periodic monitoring for hepatitis and bone marrow function (complete blood counts, platelet counts) should be performed. Because of its structure, sulfasalazine should not be prescribed for patients with sulfa allergy. Sulfasalazine is discussed in Chapter 64.
Leflunomide
Actions and Uses
Leflunomide [Arava] is a powerful immunosuppressant indicated for adults with active RA. In clinical trials, the drug decreased signs and symptoms and slowed disease progression. Compared with methotrexate, leflunomide is about equally effective but is potentially more hazardous and more expensive. Accordingly, the drug is often reserved for second-line use.
Leflunomide is a prodrug that undergoes conversion to its active form—metabolite 1 (M1)—in the body. M1 inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme needed for de novo synthesis of pyrimidines, which in turn are needed for T-cell proliferation and antibody production. In vitro, leflunomide inhibits T-cell proliferation. In animals, it suppresses inflammation.
Pharmacokinetics
After oral dosing, leflunomide is converted to M1 by enzymes in the intestine and liver. Levels of M1 peak in 6 to 12 hours. The active form undergoes further metabolism followed by excretion in the urine and bile. The half-life is prolonged: 16.5 days. As a result, a series of loading doses is needed to achieve steady state quickly.
Adverse Effects
The most common adverse effects occur in at least 10% of patients: diarrhea, respiratory infection, reversible alopecia, and rash. The drug has also been associated with much more serious reactions: pancytopenia, Stevens-Johnson syndrome, and severe hypertension.
Leflunomide is hepatotoxic. Elevation of liver enzymes occurs in about 10% of patients. In postmarketing reports, the drug has been associated with more than 130 cases of severe liver injury, including 14 that were fatal. Liver function should be assessed at baseline, every month for the first 6 months of treatment, and every 6 to 8 weeks thereafter. Leflunomide should be avoided in patients with liver impairment, hepatitis B, or hepatitis C. Patients should be informed about signs of liver injury—abdominal pain, fatigue, dark urine, and jaundice—and advised to report them immediately.
Leflunomide may increase the risk for serious infection. The drug is immunosuppressive and can suppress the bone marrow. Rarely, patients experience sepsis and other severe infections, including tuberculosis. Deaths have occurred. If an infection develops, it may be necessary to interrupt leflunomide use. To reduce risk, platelet counts and blood cell counts should be conducted at baseline, every month for the first 6 months of treatment, and every 6 to 8 weeks thereafter. If evidence of bone marrow suppression is detected, leflunomide should be discontinued. Patients should be screened for tuberculosis before starting this drug.
Leflunomide is carcinogenic in animals but has not been associated with cancer in humans.
Leflunomide and Pregnancy
Leflunomide is contraindicated during pregnancy. Patients who wish to become pregnant must first clear leflunomide from the body. A three-step protocol is followed:
Step 1: Discontinue leflunomide.
Step 2: Take cholestyramine (8 g 3 times a day) for 11 days. (Cholestyramine binds leflunomide and its metabolites in the intestine, accelerating their excretion. Without cholestyramine, safe levels might not be achieved for 2 years.)
To minimize any risk for fetal injury, men using leflunomide who wish to father a child should undergo the same clearance procedure.
Drug Interactions
Leflunomide can inhibit the metabolism of certain NSAIDs (e.g., ibuprofen, diclofenac), causing their levels to rise. In addition, leflunomide can intensify liver damage from other hepatotoxic drugs (e.g., methotrexate) and hence should not be combined with such agents. Rifampin (a drug for tuberculosis) can raise leflunomide levels by 40%. Conversely, two other agents—cholestyramine and activated charcoal—can rapidly lower leflunomide levels.
Hydroxychloroquine
Hydroxychloroquine [Plaquenil], a drug with antimalarial actions, is considered a preferred DMARD in the 2015 ACR treatment guidelines. How hydroxychloroquine works in RA is unknown. As a rule, the drug is usually combined with methotrexate. By itself, hydroxychloroquine does not slow disease progression, but early use can improve long-term outcomes.
Hydroxychloroquine should be taken with food or milk. Concurrent therapy with antiinflammatory agents (NSAIDs or glucocorticoids) is indicated during the latency period.
Retinal damage, which is rare, is the most serious toxicity. Retinopathy may be irreversible and can produce blindness. Visual loss is directly related to dosage. Low doses may be used in long-term treatment with little risk. When dosage has been excessive, retinal damage may appear after treatment has ceased and may progress in the absence of continued drug use. Patients should undergo a thorough ophthalmologic examination before treatment and every 6 months thereafter. Hydroxychloroquine should be discontinued at the first sign of retinal injury. Patients should be advised to contact the prescriber if any visual disturbance is noted.
Other Nonbiologic Disease-Modifying Antirheumatic Drugs
Several drugs that are FDA approved for RA are used infrequently, largely because of adverse effects. Some (azathioprine, cyclosporine, minocycline, and gold) are no longer recommended; however, they may still be used as a last resort when other drugs fail to meet therapeutic objectives. These drugs are discussed briefly here.
Penicillamine
Penicillamine [Cuprimine, Depen] can relieve symptoms of RA and can delay disease progression. Unfortunately, treatment may be associated with serious toxicity, especially bone marrow suppression and autoimmune disorders. Because it is associated with fatalities, penicillamine use should be restricted to cases where RA is severe and unresponsive to other treatment. Therapeutic effects take 3 to 6 months to develop.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Black Box Warning: Methotrexate [Rheumatrex, Trexall]
Black Box Warning: Methotrexate [Rheumatrex, Trexall]