Pathophysiology of Chronic Obstructive Pulmonary Disease
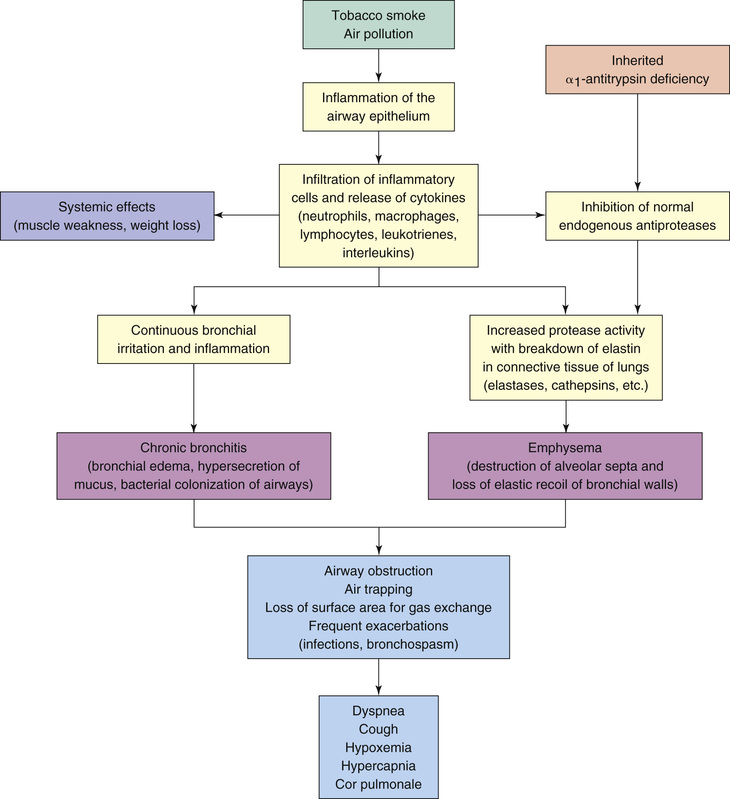
Symptoms of COPD result largely from two pathologic processes: chronic bronchitis and emphysema. In most cases, both processes are caused by an exaggerated inflammatory reaction to cigarette smoke. Chronic bronchitis—defined by chronic cough and excessive sputum production—results from hypertrophy of mucus-secreting glands in the epithelium of the larger airways. Emphysema is defined as enlargement of the air space within the bronchioles and alveoli brought on by deterioration of the walls of these air spaces. Among individuals with COPD, the relative contribution of these two processes can vary. That is, some patients may suffer primarily from chronic bronchitis, some primarily from emphysema, and some from both disease processes.
Fig. 60.2 depicts the events that lead to inflammation, airway obstruction, and air trapping in patients with COPD. Irritants such as tobacco smoke initiate an inflammatory response in the airways. As a result of the frequent and recurrent irritation and the subsequent response by various leukocytes and inflammatory mediators, pathologic changes result in the bronchial edema and increase in mucus secretion that characterize chronic bronchitis. Additionally, the continuous inflammation inhibits the production of protease inhibitors, which have a protective role in maintaining alveolar integrity. As a result of the inhibition, the protease enzymes break down elastin, resulting in the destruction of alveolar walls and the decrease in elastic recoil that characterize emphysema. In a small percentage of the population, emphysema results from a genetic alteration that results in alpha-1 antitrypsin deficiency. (Alpha-1 antitrypsin is a protease inhibitor that protects the lungs from enzymatic destruction by proteases.)
Overview of Drugs for Asthma and Chronic Obstructive Pulmonary Disease
The major drugs for asthma and COPD are shown in Table 60.1. They fall into two main pharmacologic classes: antiinflammatory agents and bronchodilators. The principal antiinflammatory drugs are the glucocorticoids. The principal bronchodilators are the beta2 agonists. For chronic asthma and stable COPD, glucocorticoids are administered on a fixed schedule, almost always by inhalation. Beta2 agonists may be administered on a fixed schedule for long-term control or as needed (PRN) to manage an acute attack. Like the glucocorticoids, beta2 agonists are usually inhaled.
TABLE 60.1
Overview of Major Drugs for Asthma and Chronic Obstructive Pulmonary Disease
ANTIINFLAMMATORY DRUGS
Glucocorticoids
Inhaled
Beclomethasone dipropionate [QVAR]
Budesonide [Pulmicort Flexhaler, Pulmicort Respules, Pulmicort Turbuhaler  ]
]
Ciclesonide [Alvesco]
Flunisolide [Aerospan]
Fluticasone propionate [Flovent HFA, Flovent Diskus]
Mometasone furoate [Asmanex Twisthaler]
Oral
Methylprednisolone [A-Methapred, Depo-Medrol, Medrol, Medrol Dose-Pak]
Prednisolone [Flo-Pred, Orapred ODT, Millipred, Pediapred, Prelone, Hydeltra TBA  ]
]
Prednisone [Deltasone, Winpred  ]
]
Leukotriene Modifiers
Montelukast, oral [Singulair]*
Zafirlukast, oral [Accolate]*
Zileuton, oral [Zyflo, Zyflo CR]*
Cromolyn
Cromolyn, inhaled [generic]*
IgE Antagonist
Omalizumab, subcutaneous [Xolair]*
Phosphodiesterase-4 Inhibitors
Roflumilast, oral [Daliresp, Daxas  ]
]
BRONCHODILATORS
Beta2-Adrenergic Agonists
Inhaled: Short Acting
Albuterol [ProAir HFA, ProAir RespiClick, Proventil HFA, Ventolin HFA, Airomir  , Apo-Salvent MDI
, Apo-Salvent MDI  ]
]
Levalbuterol [Xopenex, Xopenex HFA]
Inhaled: Long Acting‡
Arformoterol [Brovana]†
Formoterol [Foradil Aerolizer, Perforomist, Oxeze Turbuhaler  ]‡
]‡
Indacaterol [Arcapta Neohaler, Onbrez Breezhaler  ]†
]†
Olodaterol [Striverdi Respimat]†
Salmeterol [Serevent Diskus]‡
Oral
Albuterol [VoSpire ER]
Terbutaline (generic only)
Methylxanthines
Aminophylline, oral [generic]
Theophylline, oral [Theo-24, Elixophyllin, Theolair  , Theolair-SR, Pulmophylline, Theo ER
, Theolair-SR, Pulmophylline, Theo ER  ]
]
Anticholinergics
Aclidinium bromide, inhaled [Tudorza Pressair]†
Glycopyrronium bromide, inhaled [Seebri Neohaler, Seebri Breezhaler  ]†
]†
Ipratropium, inhaled [Atrovent HFA]
Tiotropium, inhaled [Spiriva, Spiriva HandiHaler, Spiriva Respimat]
Umeclidinium, inhaled [Incruse Ellipta]†
ANTIINFLAMMATORY/BRONCHODILATOR COMBINATIONS
Budesonide/formoterol, inhaled [Symbicort]
Fluticasone/salmeterol, inhaled [Advair Diskus, Advair HFA]
Fluticasone/vilanterol, inhaled [Breo Ellipta]
Mometasone/formoterol, inhaled [Dulera, Zenhale  ]
]
BETA AGONIST/CHOLINERGIC ANTAGONIST COMBINATIONS
Albuterol/ipratropium, inhaled [Combivent Respimat, Combivent UDV  ]†
]†
Indacaterol/glycopyrronium, inhaled [Utibron Neohaler, Ultibro Breezhaler  ]†
]†
Olodaterol/tiotropium, inhaled [Stiolto Respimat]†
Vilanterol/umeclidinium, inhaled [Anoro Ellipta]†
Administering Drugs by Inhalation
Most antiasthma drugs can be administered by inhalation. This route has three advantages: (1) therapeutic effects are enhanced by delivering drugs directly to their site of action, (2) systemic effects are minimized, and (3) relief of acute attacks is rapid. Three types of inhalation devices are usually employed: metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and nebulizers. Some pharmaceutical companies also have developed specialized inhaler devices for their products.
Metered-Dose Inhalers
MDIs are small, hand-held, pressurized devices that deliver a measured dose of drug with each actuation. Dosing is usually accomplished with 1 or 2 inhalations. When 2 inhalations are needed, an interval of at least 1 minute should separate the first inhalation from the second.
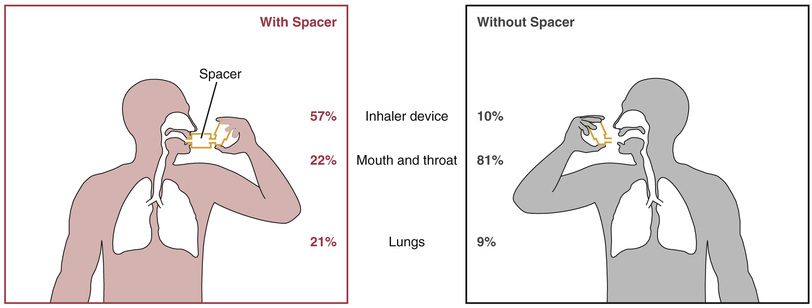
When using most MDIs, the patient must begin to inhale before activating the device. This requires hand-breath coordination, making MDIs difficult to use correctly. Accordingly, patients will need a demonstration as well as written and verbal instruction. Even with optimal use, only about 10% of the dose reaches the lungs. About 80% effects the oropharynx and is swallowed, and the remaining 10% is left in the device or exhaled.
Spacers are devices that attach directly to the MDI to increase delivery of drug to the lungs and decrease deposition of drug on the oropharyngeal mucosa (Fig. 60.3). Several kinds of spacers are available for use with MDIs. Some spacers contain a one-way valve that activates on inhalation, obviating the need for good hand-breath coordination. Some spacers also contain an alarm whistle that sounds off when inhalation is too rapid, thus maximizing effective drug administration. They can also prevent bronchospasm that may occur with sudden intake of an inhaled drug.
Dry-Powder Inhalers
DPIs are used to deliver drugs in the form of a dry, micronized powder directly to the lungs. Unlike MDIs, DPIs are breath activated. As a result, DPIs don’t require the hand-breath coordination needed with MDIs, and so DPIs are much easier to use. Compared with MDIs, DPIs deliver more drug to the lungs (20% of the total released vs. 10%) and less to the oropharynx. Also, spacers are not used with DPIs.
Nebulizers
A nebulizer is a small machine used to convert a drug solution into a mist. The droplets in the mist are much finer than those produced by inhalers, resulting in less drug deposit on the oropharynx and increased delivery to the lung. Inhalation of the nebulized mist can be done through a face mask or through a mouthpiece held between the teeth. Because the mist produced by a nebulizer is inhaled with each breath, hand-breath coordination is not a concern. Nebulizers take several minutes to deliver the same amount of drug contained in 1 inhalation from an inhaler, but for some patients, a nebulizer may be more effective than an inhaler. Although nebulizers are usually used at home or in a clinic or hospital, these devices, which weigh less than 10 pounds, are sufficiently portable for use in other locations.
Antiinflammatory Drugs
Antiinflammatory drugs—especially inhaled glucocorticoids—are the foundation of asthma and COPD therapy. These drugs are taken daily for long-term control. Most people with asthma require these drugs for management at some point.
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Antiinflammatory Agents
| Life Stage | Patient Care Concerns |
| Children | Inhaled glucocorticoids are the preferred long-term treatment for children of all ages, including infants. Face masks are recommended for administration of inhaled glucocorticoids to children younger than 4 years. Alternative treatments include cromolyn and leukotriene receptor antagonists (e.g., montelukast), but evidence supporting these drugs for asthma management is lower than that supporting inhaled glucocorticoids. Montelukast is the only leukotriene modifier approved for children aged 1–5 years. |
| Pregnant women | Inhaled glucocorticoids are classified in FDA Pregnancy Risk Category C; however, they are preferred for uncontrolled asthma in pregnant women because uncontrolled asthma is associated with greater fetal risks. Of the leukotriene modifiers, montelukast and zafirlukast are Pregnancy Risk Category B, whereas zileuton is Pregnancy Risk Category C. |
| Breastfeeding women | Inhaled glucocorticoids are not a contraindication to breastfeeding. Women taking systemic glucocorticoids should not breastfeed. |
| Older adults | Benefits exceed risk. Inhaled glucocorticoids are much safer than systemic formulations. |
Glucocorticoids
Glucocorticoids (e.g., budesonide, fluticasone) are the most effective drugs available for long-term control of airway inflammation. Administration is usually by inhalation, but may also be intravenous (IV) or oral. Adverse reactions to inhaled glucocorticoids are generally minor, as are reactions to systemic glucocorticoids taken acutely. However, when systemic glucocorticoids are used long term, severe adverse effects are likely. The basic pharmacology of the glucocorticoids is presented in Chapter 56. Discussion here is limited to their use in asthma.
Mechanism of Antiasthma Action
Glucocorticoids reduce asthma symptoms by suppressing inflammation. Specific antiinflammatory effects include the following:
• Decreased synthesis and release of inflammatory mediators (e.g., leukotrienes, histamine, prostaglandins)
• Decreased infiltration and activity of inflammatory cells (e.g., eosinophils, leukocytes)
• Decreased edema of the airway mucosa (secondary to a decrease in vascular permeability)
By suppressing inflammation, glucocorticoids reduce bronchial hyperreactivity and decrease airway mucus production. There is also some evidence that glucocorticoids may increase the number of bronchial beta2 receptors as well as their responsiveness to beta2 agonists.
Use in Asthma
Glucocorticoids are used for prophylaxis in managing chronic asthma; therefore, dosing must be done on a fixed schedule—not PRN. Because beneficial effects develop slowly, these drugs cannot be used to abort an ongoing attack. Glucocorticoids do not alter the natural course of asthma, even when used in young children; however, they provide significant long term control and management of symptoms.
Inhalation Use
Inhaled glucocorticoids are first-line therapy for management of the inflammatory component of asthma. Most patients with persistent asthma should use these drugs daily. Inhaled glucocorticoids are very effective and are much safer than systemic glucocorticoids.
Oral Use
Oral glucocorticoids may be required for patients with moderate to severe persistent asthma or for management of acute exacerbations of asthma or COPD. Because of their potential for toxicity, these drugs are prescribed only when symptoms cannot be controlled with safer medications (inhaled glucocorticoids, inhaled beta2 agonists). Because the risk for toxicity increases with duration of use, treatment should be as brief as possible.
Adverse Effects
Inhaled Glucocorticoids
These preparations are largely devoid of serious toxicity, even when used in high doses. The most serious concern is adrenal suppression.
The most common adverse effects are oropharyngeal candidiasis and dysphonia (hoarseness, speaking difficulty). Both effects result from local deposition of inhaled glucocorticoids. To minimize these effects, patients should rinse the mouth with water and gargle after each administration. Using a spacer device can help too. If candidiasis develops, it can be treated with an antifungal drug.
With long-term, high-dose therapy, some adrenal suppression may develop, although the degree of suppression is generally low. In contrast, with prolonged use of oral glucocorticoids, adrenal suppression can be profound.
Glucocorticoids can slow growth in children and adolescents—but these drugs do not decrease adult height. Short-term studies have shown that inhaled glucocorticoids slow growth; however, long-term studies indicate that adult height, although delayed, is not reduced. Less is known regarding whether glucocorticoids suppress growth and development of the brain, lungs, and other organs, in part because having asthma alone can affect organ growth. Because the benefits of inhaled glucocorticoids tend to be much greater than the risks, current guidelines for asthma management recommend these drugs for children while monitoring for evidence of complications.
Long-term use of inhaled glucocorticoids may promote bone loss. Fortunately, the amount of loss is much lower than the amount caused by oral glucocorticoids. To minimize bone loss, patients should (1) use the lowest dose that controls symptoms, (2) ensure adequate intake of calcium and vitamin D, and (3) participate in weight-bearing exercise.
There has been concern that prolonged therapy might increase the risk for cataracts and glaucoma. Although this may be an issue of concern with continuous use of high-dose inhaled glucocorticoids, this problem is not associated with long-term use of low to medium doses of inhaled glucocorticoids.
Oral Glucocorticoids
When used acutely (less than 10 days), even in very high doses, oral glucocorticoids do not cause significant adverse effects. However, prolonged therapy, even in moderate doses, can be hazardous. Potential adverse effects include adrenal suppression, osteoporosis, hyperglycemia, peptic ulcer disease, and, in young patients, growth suppression.
Adrenal suppression is of particular concern. As discussed in Chapter 56, prolonged glucocorticoid use can decrease the ability of the adrenal cortex to produce glucocorticoids of its own. This can be life-threatening at times of severe physiologic stress (e.g., surgery, trauma, or systemic infection). Because high levels of glucocorticoids are required to survive severe stress and because adrenal suppression prevents production of endogenous glucocorticoids, patients must be given increased doses of oral or IV glucocorticoids at times of stress. Failure to do so can prove fatal.
Compensating for Adrenal Insufficiency
When patients have been on prolonged systemic glucocorticoid therapy, the adrenal glands decrease their endogenous production of glucocorticoids. If systemic therapy is stopped suddenly, as when switching from oral therapy to inhalation therapy, the patient can die. Similarly, during times of severe physical stress when the body would normally produce high levels of glucocorticoids, if the dose of systemic glucocorticoids is not increased to compensate, the patient can die. What important lesson can you take from this? When discontinuing a systemic glucocorticoid, you must be sure it is done gradually to allow the body to resume producing the endogenous hormone. On the other hand, if a patient taking systemic glucocorticoids experiences severe physical stress, such as a motor vehicle crash, or is scheduled for a stressful procedure, such as surgery, you must prescribe additional glucocorticoids to supplement for the endogenous hormone that the patient cannot produce.
Adrenal suppression is also a concern when discontinuing prolonged use of oral glucocorticoids or when transferring from an oral route to an inhaled route. Several months are required for recovery of adrenocortical function, so it is important to decrease the dosage gradually. Throughout this time, all patients—including those switched to inhaled glucocorticoids—must be given supplemental oral or IV glucocorticoids at times of severe stress.
A complete list of contraindications to oral glucocorticoids is presented in the “Prescribing and Monitoring Considerations” section at the end of this chapter.
Preparations, Dosage, and Administration
Inhaled Glucocorticoids
Six glucocorticoids are available for inhalation (Table 60.2). Four are available in MDIs, three are available in DPIs, and one is available in suspension for nebulization. Inhaled glucocorticoids are administered on a regular schedule—not PRN. Pediatric and adult dosages are shown in Table 60.2. The dosage should be kept as low as possible to minimize adrenal suppression, possible bone loss, and other adverse effects.
TABLE 60.2
Inhaled Glucocorticoids: Formulations and Dosages
| Dosage | |||
| Drug | Formulation | Adults | Children |
| Beclomethasone dipropionate [QVAR] | MDI: 40 or 80 mcg/inhalation | 40–320 mcg twice daily | 40–80 mcg twice daily (5–11 years) |
| Budesonide | |||
| [Pulmicort Flexhaler] | DPI: 90 or 180 mcg/inhalation | 360–720 mcg twice daily | 180–360 mcg twice daily (6–17 years) |
| [Pulmicort Respules] | Suspension for nebulization | 250–500 mcg once or twice daily or 1000 mcg once daily | 500–1000 mcg/day (1–8 years) |
| Ciclesonide [Alvesco] | MDI: 80 or 160 mcg/inhalation | 80–320 mcg twice daily | 80–320 mcg twice daily (12 years and up) |
| Flunisolide [Aerospan] | MDI: 80 mcg/inhalation | 160–320 mcg twice daily | 80–320 mcg twice daily (6–11 years) |
| Fluticasone propionate | |||
| [Flovent HFA] | MDI: 44, 110, or 220 mcg/inhalation | 88–440 mcg twice daily | 88 mcg twice daily (4–11 years) |
| [Flovent Diskus] | DPI: 50, 100, or 250 mcg/inhalation | 100–1000 mcg twice daily | 50–100 mcg twice daily (4–11 years) |
| Mometasone furoate [Asmanex Twisthaler] | DPI: 110 or 220 mcg/inhalation | 220–440 mcg once or twice daily | 110 mcg once daily (4–11 years) |
Nebulized Budesonide
Budesonide suspension [Pulmicort Respules] is the first inhaled glucocorticoid formulated for nebulized dosing. The product is approved for maintenance therapy of persistent asthma in children 1 to 8 years old. Improvement should begin in 2 to 8 days; maximal benefits may take 4 to 6 weeks to develop. Budesonide suspension is available in 2-mL ampules containing 250 or 500 mcg of the drug. Administration is done with a jet nebulizer equipped with a mouthpiece or face mask; ultrasonic nebulizers should not be used. Administration takes 5 to 10 minutes. For children who are not taking an oral glucocorticoid, the initial dosage is 500 mcg/day in one or two doses. For children who are taking an oral glucocorticoid, the initial dosage is 1000 mcg/day in one or two doses. After 1 week, dosage of the oral glucocorticoid should be tapered off.
Oral Glucocorticoids
Methylprednisolone, prednisone, and prednisolone are preferred glucocorticoids for oral therapy of asthma. The dosage is the same regardless of the drug.
When beginning therapy with oral glucocorticoids, dosing initially focuses on bringing symptoms under control. The National Asthma Education and Prevention Program (NAEPP) guidelines recommend an initial burst of 40 to 60 mg administered daily for 3 to 10 days for adults. The initial pediatric dosage is 1 to 2 mg/kg/day for 3 to 10 days. Thereafter, the typical dose is 0.25 to 2 mg/kg daily or every other day for children younger than 12 years and 7.5 to 60 mg daily or every other day for older children and adults. For long-term treatment, alternate-day dosing is recommended to minimize adrenal suppression. After symptoms have been controlled for 3 months, dosages should be decreased gradually to establish the lowest dosage that can keep the patient free of symptoms. As discussed previously, the dosage of oral glucocorticoids must be increased during times of stress.
Leukotriene Modifiers
Leukotrienes modifiers suppress the effects of leukotrienes, which are compounds that promote smooth muscle constriction, blood vessel permeability, and inflammatory responses through direct action as well as through recruitment of eosinophils and other inflammatory cells. In patients with asthma, these drugs can decrease bronchoconstriction and inflammatory responses such as edema and mucus secretion.
Three leukotriene modifiers are currently available: zileuton, zafirlukast, and montelukast. Zileuton blocks leukotriene synthesis; zafirlukast and montelukast block leukotriene receptors. All three drugs are dosed orally. Current guidelines recommend using these agents as second-line therapy (if an inhaled glucocorticoid cannot be used) and as add-on therapy when an inhaled glucocorticoid alone is inadequate. Although generally well tolerated, all of the leukotriene modifiers can cause adverse neuropsychiatric effects, including depression, suicidal thinking, and suicidal behavior.
Zileuton
Zileuton [Zyflo, Zyflo CR], an inhibitor of leukotriene synthesis, is approved for asthma prophylaxis and maintenance therapy in adults and children 12 years and older. Symptomatic improvement can be seen within 1 to 2 hours of dosing. Because effects are not immediate, zileuton cannot be used to abort an ongoing attack. Zileuton is less effective than an inhaled glucocorticoid alone and appears to be less effective than a long-acting inhaled beta2 agonist as adjunctive therapy in patients not adequately controlled with an inhaled glucocorticoid.
Mechanism of Action
Benefits derive from inhibiting 5-lipoxygenase, the enzyme that converts arachidonic acid into leukotrienes. This decreases the amount of leukotrienes available to induce inflammation.
Pharmacokinetics
Zileuton is given orally and undergoes rapid absorption, in both the presence and absence of food. Plasma levels peak 2 to 3 hours after dosing. Zileuton is rapidly metabolized by the liver, and the metabolites are excreted in the urine. Its plasma half-life is 2.5 hours.
Adverse Effects
Zileuton can injure the liver, as evidenced by increased plasma levels of alanine aminotransferase (ALT) activity. A few patients have developed symptomatic hepatitis, which reversed after drug withdrawal. To reduce the risk for serious liver injury, ALT activity should be monitored. The recommended schedule is once a month for 3 months, then every 2 to 3 months for the remainder of the first year, and periodically thereafter.
Postmarketing reports indicate that zileuton and the other leukotriene modifiers can cause adverse neuropsychiatric effects, including depression, anxiety, agitation, abnormal dreams, hallucinations, insomnia, irritability, restlessness, and suicidal thinking and behavior. If these develop, switching to a different medication should be considered.
Zileuton is metabolized by cytochrome P450, where it acts as an inhibitor of CYP1A2 isoenzymes and can slow metabolism of drug substrates metabolized by this pathway, increasing their levels. Combined use with theophylline can markedly increase theophylline levels, so dosage of theophylline should be reduced. Zileuton can also increase levels of warfarin and propranolol.
Preparations, Dosage, and Administration
Zileuton is available in 600-mg immediate-release tablets, sold as Zyflo, and 600-mg extended-release tablets, sold as Zyflo CR. With the immediate-release tablets, the recommended dosage is 600 mg 4 times a day. With the extended-release tablets, the recommended dosage is two 600 mg tablets twice a day, taken within 1 hour of the morning and evening meals.
Zafirlukast
Zafirlukast [Accolate] was the first representative of a unique group of antiinflammatory agents, the leukotriene receptor antagonists. The drug is approved for maintenance therapy of chronic asthma in adults and children 5 years and older.
Mechanism of Action
Benefits derive in part from reduced infiltration of inflammatory cells, resulting in decreased bronchoconstriction.
Pharmacokinetics
Zafirlukast is administered orally, and absorption is rapid. Food reduces absorption by 40%; therefore, the drug should be administered at least 1 hour before meals or 2 hours after. Zafirlukast undergoes hepatic metabolism followed by fecal excretion. The half-life is about 10 hours but may be as long as 20 hours in older adults.
Adverse Effects
The most common side effects of zafirlukast are headache and gastrointestinal (GI) disturbances, both of which are infrequent. Arthralgia and myalgia may also occur. Like zileuton, zafirlukast can cause depression, suicidal thinking, hallucinations, and other neuropsychiatric effects. A few patients have developed Churg-Strauss syndrome, a potentially fatal disorder characterized by weight loss, flu-like symptoms, and pulmonary vasculitis (blood vessel inflammation). However, in most cases, symptoms developed when glucocorticoids were being withdrawn, suggesting that glucocorticoid withdrawal may be a contributing factor.
Rarely, patients develop clinical signs of liver injury (e.g., abdominal pain, jaundice, fatigue). If these occur, zafirlukast should be discontinued, and liver function tests (especially serum ALT) should be performed immediately. If test results are consistent with liver injury, zafirlukast should not be resumed. Curiously, signs of liver injury have developed mainly in females.
Zafirlukast inhibits several isoenzymes of cytochrome P450 and can suppress metabolism of other drugs, causing their levels to rise. Concurrent use can raise serum theophylline to toxic levels. Theophylline levels should be closely monitored, especially when zafirlukast is started or stopped. Zafirlukast can also raise levels of warfarin (an anticoagulant) and thus may cause bleeding.
Preparations, Dosage, and Administration
Zafirlukast is available in 10- and 20-mg tablets. The dosage for adults and children 12 years and older is 20 mg twice a day. The dosage for children age 5 to 11 years is 10 mg twice a day. Zafirlukast should not be administered with food.
Montelukast
Montelukast [Singulair], a leukotriene receptor blocker, is the most commonly used leukotriene modulator. The drug has three approved indications: (1) prophylaxis and maintenance therapy of asthma in patients at least 1 year old; (2) prevention of exercise-induced bronchospasm (EIB) in patients at least 15 years old; and (3) relief of allergic rhinitis (see Chapter 61). Montelukast cannot be used for quick relief of an asthma attack because effects develop too slowly. For prophylaxis and maintenance therapy of asthma, maximal effects develop within 24 hours of the first dose and are maintained with once-daily dosing in the evening. In clinical trials, montelukast decreased asthma-related nocturnal awakening, improved morning lung function, and decreased the need for a short-acting inhaled beta2 agonist throughout the day. Although montelukast is approved for preventing EIB, a short-acting beta2 agonist is preferred.
Mechanism of Action
Montelukast has a high affinity for leukotriene receptors in the airway and on proinflammatory cells such as eosinophils. By occupying these receptors, the drug blocks receptor activation by the body’s leukotrienes.
Pharmacokinetics
Montelukast is rapidly absorbed after oral administration. Bioavailability is about 64%. Blood levels peak 3 to 4 hours after ingestion. The drug is highly bound (more than 99%) to plasma proteins. Montelukast undergoes extensive metabolism by hepatic cytochrome P450 enzymes followed by excretion in the bile. The plasma half-life ranges from 2.7 to 5.5 hours.
Adverse Effects
Montelukast is generally well tolerated. In clinical trials, adverse effects were equivalent to those of placebo. In contrast to zileuton and zafirlukast, montelukast does not seem to cause liver injury. As with zafirlukast, Churg-Strauss syndrome has occurred when glucocorticoid dosage was reduced. Postmarketing reports suggest a link between montelukast and neuropsychiatric effects, especially mood changes and suicidality. Fortunately, these effects are rare.
Montelukast appears devoid of serious drug interactions. Unlike zileuton and zafirlukast, it does not increase levels of theophylline or warfarin. Concurrent use of phenytoin (an anticonvulsant that induces P450 isoenzymes) can decrease levels of montelukast.
Preparations, Dosage, and Administration
Montelukast is available in three formulations: standard tablets (10 mg), chewable tablets (4 and 5 mg), and oral granules (4 mg/packet). The oral granules may be put directly in the mouth or may be mixed with one spoonful of either applesauce, carrots, rice, or ice cream. For prophylaxis or chronic treatment of asthma, dosing is done once a day in the evening, with or without food. Dosage is based on patient age as follows:
• Age 15 years and older—one 10-mg tablet daily
• Age 6 to 14 years—one 5-mg chewable tablet daily
• Age 2 to 5 years—one 4-mg chewable tablet or 4 mg of oral granules daily
To prevent EIB, patients should take one 10-mg tablet at least 2 hours before exercising. No additional dose should be taken for at least 24 hours. Patients already taking montelukast daily should not take any more to prevent EIB.
Cromolyn
Cromolyn is an inhalational agent that suppresses bronchial inflammation. The drug is used for prophylaxis—not quick relief—in patients with mild to moderate asthma. Antiinflammatory effects are less than with glucocorticoids; therefore, cromolyn is not a preferred drug for asthma therapy. When glucocorticoids create problems, however, cromolyn may be prescribed as alternative therapy.
Mechanism of Action
Cromolyn suppresses inflammation; it does not cause bronchodilation. The drug acts in part by stabilizing the cytoplasmic membrane of mast cells, preventing release of histamine and other mediators. In addition, cromolyn inhibits eosinophils, macrophages, and other inflammatory cells.
Pharmacokinetics
Cromolyn is administered by nebulizer. The fraction absorbed from the lungs is small and rarely produces significant systemic effects. Absorbed cromolyn is excreted unchanged in the urine.
Therapeutic Uses
Chronic Asthma
Cromolyn is an alternative to inhaled glucocorticoids for prophylactic therapy of mild persistent asthma. When administered on a fixed schedule, cromolyn reduces both the frequency and intensity of asthma attacks. Maximal effects may take several weeks to develop. No tolerance to effects is seen with long-term use. Cromolyn is especially effective for prophylaxis of seasonal allergic attacks and for acute allergy prophylaxis immediately before allergen exposure (e.g., before mowing the lawn).
Allergic Rhinitis
Intranasal cromolyn [NasalCrom] can relieve symptoms of allergic rhinitis (see Chapter 61).
Adverse Effects
Cromolyn is the safest of all antiasthma medications. Significant adverse effects occur in fewer than 1 of every 10,000 patients. Occasionally, cough or bronchospasm occurs in response to cromolyn inhalation.
Preparations, Dosage, and Administration
Cromolyn is administered using a power-driven nebulizer. The initial dosage for adults and children is 20 mg 4 times a day. For maintenance therapy, the lowest effective dosage should be established.
Omalizumab
Omalizumab [Xolair] is a monoclonal antibody with a unique mechanism of action: antagonism of IgE, a type of antibody. The drug is a second-line agent indicated only for allergy-related asthma and only when preferred options have failed. Omalizumab offers modest benefits and has significant drawbacks: the drug poses a risk for anaphylaxis and cancer, must be given subcutaneously, and costs more than $10,000 a year. Furthermore, its long-term safety is unknown.
Mechanism of Action
Omalizumab forms complexes with free IgE in the blood and thereby reduces the amount of IgE available to bind with its receptors on mast cells. This greatly reduces the number of IgE molecules on the mast cell surface and thus limits the ability of allergens to trigger release of histamine, leukotrienes, and other mediators that promote bronchospasm and airway inflammation. At recommended doses, omalizumab decreases free IgE in serum by 96%. When treatment stops, about 1 year is required for free IgE to return to its pretreatment level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree