Drug-induced alopecia
Idiopathic alopecia
Temporal association of drug initiation and alopecia
No association with initiation of drug
Cessation of drug leads to recovery and restoration of hairs and reversion of histopathological changes
No recovery of hairs after cessation of drug
No evidence of thyroid dysfunction
± Thyroid dysfunction
Drugs Implicated
Anti-coagulants
Many anti-coagulants are known to cause reversible hair loss, but the exact mechanism and pathogenesis remain unclear. Systemic heparin and heparinoid therapy can cause transitory diffuse hair loss, namely telogen effluvium, in up to 50 % of patients. Animal models have demonstrated that heparin has an anti-mitotic effect on follicular epithelial cells, inhibits anagen growth, stimulates epidermal proliferation, and inhibits epithelial bulb cell proliferation. Hair re-growth characteristically occurs after drug cessation. Low-molecular-weight heparins (LMWHs) are a mixture of short-chain heparins (2,000–10,000 Da) and are safer than unfractionated heparins. Additionally, LMWHs have fewer complications, can be given in discrete doses, and do not require drug monitoring. Dalteparin was the first reported LMWH to cause rapid, diffuse, reversible alopecia. The first report was 10 weeks after dalteparin treatment for sinus thrombosis in a 9-year-old girl and the second case series reported this phenomenon in hemodialysis patients. Hair re-growth commenced nearly 6 weeks after discontinuation of dalteparin. Barnes et al. demonstrated that hair re-growth can be re-established with citrate anticoagulation. Wang et al. reported three cases of alopecia in women after initiation of enoxaparin for central venous and sinus thrombosis. In these cases, telogen effluvium was precipitated by enoxaparin-induced premature transformation of anagen-phase follicles into telogen-phase, resting follicles. Tinzaparin is another LMWH associated with reversible hair loss, which has been documented in a 66-year-old patient on hemodialysis. The effect and severity of LMWH-associated hair loss is thought to be related to the dose and not to the duration of therapy, and there is typically a latent period of 2 weeks from the time of drug administration to hair shedding. Warfarin is less likely to cause clinically significant alopecia, although it has been documented (Fig. 20.1). According to Flesch, the onset of warfarin-induced alopecia may be extremely delayed, often up to years. Three cases of warfarin-induced hair loss were reported by Umlas and Harken, which occurred many years after continuous warfarin therapy for heart disease. They also noted that the alopecia was dose independent and this phenomenon is likely to be under-diagnosed because of the late onset.
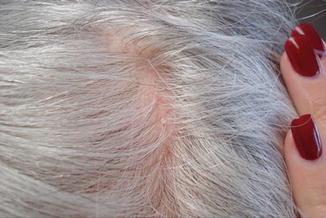

Fig. 20.1
Diffuse hair loss with over 25 % telogen hairs on trichogram that began after Coumadin therapy was initiated. Note the scalp is normal other than mild small nonadherent scaling of seborrhea
Antidepressants
Alopecia is a known side effect of antidepressant medications. Treatment consists of various regimens, including tricyclic or monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRI), or serotonin–norepinephrine reuptake inhibitor (SNRI). Few examples of alopecia induced by tricyclic antidepressants exist in the literature, but cases of hair loss associated with imipramine and desipramine have been reported. No cases of hair loss have been reported with use of MAOIs. Second-generation antidepressants, specifically SSRIs, have caused telogen effluvium. Sertraline, paroxetine, fluoxetine, and citalopram have all been shown to cause reversible alopecia with re-growth as early as 3 months following cessation of use; however, hair regrowth was delayed 1.5 years in a least one case report.
Antimicrobials
Antibiotics, antivirals, antifungals, antihelminthics, and antiretrovirals can all damage hair growth and induce not only telogen effluvium but, rarely, alopecia universalis. Antibiotics have not historically shown a strong predilection toward damaging the hair follicle. Hajime et al. reported a case linking gentamycin to scalp and eyebrow loss following treatment of pseudomonas in a 15-year-old male. Few case reports exist describing dose-related reversible alopecia secondary to nitrofurantoin. The anti-tuberculin drugs isoniazid, thiacetazone, and ethionamide have all been associated with alopecia in the past, but the mechanism is unclear. Hypotheses include involvement of androgen, as isoniazid has been shown to alter estrogen-androgen metabolism. Azole antifungal medications, namely fluconazole and itraconazole, as well as anidulafungin, have been associated with hair loss at high doses. The antihelminthics benzimidazole and albendazole are used for echinococcosis infections, and both have been reported to cause reversible alopecia.
Interferon-alpha (IFN-α), used in the treatment of Hepatitis C, causes dose-independent alopecia in 50 % of patients. Hair loss caused by IFN-α can be localized to an injection site or cause telogen effluvium and, in rare cases, can cause alopecia universalis. The alopecia associated with interferon-alpha therapy is transient and resolves following discontinuation of the drug (see the section on Interferon). Antiretroviral drugs carry a moderate risk for development of alopecia. Approximately 10 % of patients treated with indinavir will experience severe telogen effluvium, possibly with patchy hair loss of the legs, thighs, pubic, and axillary regions. Combined treatment with indinavir and ritonavir may increase the severity of adverse effects because ritonavir increases the plasma concentration of indinavir. A proposed mechanism of action for telogen effluvium associated with indinavir is the enhancement of retinoic acid signaling specific to indinavir. Transitioning to a new drug regimen or discontinuing indinavir will allow restoration of normal hair growth.
Busulfan
Busulfan is a chemotherapeutic agent commonly used in conditioning regimens prior to bone marrow transplant. Unlike most chemotherapeutic agents, which cause a reversible anagen effluvium, busulfan has been reported to cause permanent partial or diffuse hair loss in up to 50 % of patients. Scalp biopsies in these patients demonstrate decreased follicle density without associated inflammation or fibrosis. Reduced follicle density may be a consequence of stem cell destruction or acute damage to matrix keratinocytes.
Cardiovascular Drugs
Beta-blockers are commonly used to treat hypertension, but have a known side effect of alopecia (Fig. 20.2), specifically telogen effluvium. Metoprolol (Lopressor), propranolol (Inderol), and nadolol (Corgard) have been documented to cause telogen effluvium. Hair re-growth has been reported within 3 months of nadolol withdrawal. The angiotensin-converting enzyme inhibitors (ACEi), used in the treatment of hypertension and congestive heart failure, have also been associated with hair loss. In one report a combination of captopril (Capoten) and furosemide cased diffuse hair loss. Angiotensin-converting enzyme inhibitors and β-receptor blocking agents may rarely precipitate a rapidly progressive lichen planopilaris in susceptible patients. Use of these agents in patients with active lichen planopilaris should be avoided. Amiodarone, an anti-arrhythmic, is known for its numerous side effects, including alopecia. In all cases, hair re-growth was reported upon discontinuation of amiodarone.
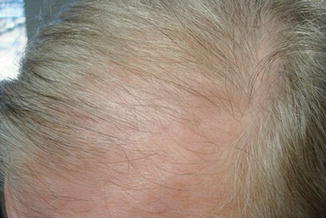

Fig. 20.2
Diffuse hair loss due to a beta blocker, which dissipated months after the drug was discontinued
Chemotherapy Agents
Chemotherapy-induced alopecia (CIA) is commonplace for patients receiving a cytotoxic drug regimen. Although it is a well-known side effect of therapy, the anticipated hair loss can be extremely distressing, enough so that patients consistently rank it among the worst hardships involved with chemotherapy. CIA can be seen within days of initiating treatment, followed by complete hair loss around the second cycle, 4–8 weeks following induction. The degree of hair loss is directly related to dose, schedule, rate, and route of delivery. Hair loss typically begins at the crown of the head, followed by the temporal region. The hair loss can be diffuse or patchy, depending on which individual follicles are in the anagen phase. Chemotherapeutic agents most commonly associated with hair loss include anthracyclines, antibiotics, antimetabolites, vinca alkaloids, and taxanes. The cytotoxic drugs used in chemotherapy target rapidly dividing cells; unfortunately, hair follicles are innocent bystanders and unintended targets. Due to the fact that the hair follicles affected are dividing, or in the anagen phase, the term used to describe CIA is anagen effluvium. It is known that p53 plays a large role in hair follicle apoptosis, and recent studies have shown that Fas and c-kit do play a role, but the exact molecular pathway is still not fully understood. An ongoing debate continues surrounding more hypothetical pathways. The vast majority of patients will have hair re-growth 1-3 months following the discontinuation or completion of chemotherapy, however, the hair texture, thickness, color, and waviness may be altered in up to 60 % of patients.
Dopaminergic Therapy
Levodopa, ergot, and non-ergot alkaloid dopamine receptor agonists have been associated with alopecia and generally affect women more than men. Case reports have documented telogen effluvium in patients with Parkinson’s disease that were taking dopaminergic medications. Levodopa, bromocriptine, pramipexole, ropinirole, cabergoline, and pergolide have all been described in the literature as having adverse effects on hair. The pathophysiologic mechanism is unknown, but switching agents or stopping treatment has been shown to reverse the adverse effects over time.
Extracorporeal Membrane Oxygenation (ECMO)
ECMO is a mechanical cardiopulmonary support device that acts as an artificial lung during cardiothoracic surgery or in patients with severe respiratory distress. Hair loss following EMCO is extremely common and reported extensively. One study demonstrated that up to 87 % of patients will potentially lose hair following utilization of ECMO. The etiology of hair loss with ECMO is most likely multifactorial and resolves spontaneously over time.
Fluoroscopy
Rarely, the use of fluoroscopy during interventional procedures, such as neurointervention following an acute stroke or coronary angiography and angioplasty, has been associated with radiation dermatitis and alopecia in exposed areas. Some authors have termed this “square alopecia,” reflecting the geometric distribution pattern frequently observed. This is commonly misdiagnosed as alopecia areata, as the patient may not readily provide a history of exposure to fluoroscopy. It most commonly occurs in the retroauricular region, as the highest dosages of radiation are frequently applied in this location. The severity is proportional to the fluoroscopy dose, the total time of the procedure, the interval between exposures, the size of the irradiated area, and individual patient characteristics, such as age, smoking status, nutritional status, skin integrity, tissue oxygenation, capillary density, hormonal status, genetic factors, obesity, and skin color.
Hormonal Therapies
The estrogen in oral contraceptive pills (OCPs) has been linked to prolonged anagen duration. An alternate hypothesis is that some OCPs contain anti-androgens (drosperidone, cyproterone acetate), which arrest androgenetic alopecia. Subsequent cessation of OCPs presumably then leads to resumption of an unrecognized androgenetic alopecia. Telogen effluvium can also be seen following cessation or interruption of OCPs. Some progesterone-based medications (levonorgestrel, norgestrel, norethisterone, tibolone) may induce or worsen androgenetic alopecia.
Human Epidermal Receptor (HER) Tyrosine Kinase Inhibitors
Tufted hair folliculitis has been reported to develop during treatment with both lapatinib (a HER1 and HER2 tyrosine kinase inhibitor) and trastuzumab (a HER2 monoclonal antibody inhibitor). In the case of trastuzumab, the patient experienced significant scaling and pruritus in association with tufted hair folliculitis. These symptoms resolved with clobetasol propionate 0.05 % topical solution applied twice daily.
The HER1 tyrosine kinase inhibitors erlotinib and gefitinib have been reported to cause a cicatricial alopecia with associated chronic folliculitis and perifolliculitis. Erlotinib has also been reported to cause folliculitis decalvans, which improved with antimicrobial and topical corticosteroid therapy despite continuation of treatment.
Interferon (IFN)
Telogen effluvium occurs in up to 50 % of patients receiving interferon therapy. This effect is not dose-related. Hair shedding is reversible after interruption of treatment and, in some cases, despite continuation of treatment. Changes in hair texture and color upon regrowth are frequently observed. For example, in one report 18 % of patients treated with low-dose IFN-α for malignant melanoma experienced hair whitening. Acquired hair straightening has also occurred in patients on combined IFN-α and ribavirin therapy for hepatitis B. Transient localized alopecia has been reported at IFN-α injection sites.
Multiple cases of localized alopecia areata, alopecia totalis, and alopecia universalis during or shortly after treatment of chronic Hepatitis C with peg-IFN-α and ribavirin have been reported in the literature. At least one case of alopecia totalis involved a patient with pre-existing alopecia areata. Alopecia was noted 3–9 months following treatment initiation, and resolution occurred irrespective of treatment in most patients 3–12 months later. There are at least two reported cases of irreversible alopecia universalis and totalis following peg IFN and ribavirin therapy.
Minoxidil
Topical minoxidil is commonly used in the treatment of alopecia, specifically androgenetic alopecia. Paradoxically, diffuse hair shedding often occurs within 4–6 weeks of initiating therapy secondary to a brief telogen effluvium. Minoxidil induces premature termination of the telogen phase, leading to simultaneous release of many telogen hairs as responding follicles transition to anagen.
Upon discontinuation of minoxidil, all of the follicles that had prolonged anagen phases during therapy simultaneously enter the telogen phase. This results in a severe telogen effluvium approximately 2–3 months later. Minoxidil has also been reported to induce hair darkening.
Mood Stabilizers
Although uncommon, psychiatric medications can cause alopecia. Mood stabilizers such as lithium and valproic acid are two of the more common inducers of telogen effluvium, but often these symptoms are dose-related and readily reversible with modification of the amount given. It has been reported that 12–19 % of long-term users of lithium will experience hair loss or hair thinning. Alopecia may occur weeks to years into treatment with lithium, with the typical time course being 4–6 months. The adverse drug effects of lithium treatment affect females more often than males. It is essential to rule out lithium-induced thyroid disease, a common side effect of lithium treatment, in patients with hair loss or thinning. Returning the patient to a euthyroid state should readily reverse the hair loss. The incidence of alopecia caused by valproic acid is 0.5–12 %. Valproic acid-associated alopecia seems to be associated with increased valproic acid blood concentration, and dose reduction allows hair regrowth. Carbamazepine therapy can also lead to hair loss, with an incidence of 1.6–6 %. Newer mood-stabilizing compounds such as gabapentin rarely cause alopecia. Among the antipsychotic drug classes, only haldoperidol, olanzapine, and rispiridone have been reported to cause hair loss.
Mycophenolate Mofetil
In a long-term observational prospective study of mycophenolate mofetil use in patients with proliferative lupus nephritis, new onset alopecia was noted in 1 of 33 patients. Due to alopecia being a known complication of systemic lupus erythematosus, these results should be interpreted with caution. As discussed below, alopecia has also been seen in patients taking combination mycophenolate mofetil and tacrolimus therapy for long-term immunosuppression following transplant.
Radiation
Radiation for the treatment of brain tumors commonly leads to cicatricial alopecia in exposed areas of the scalp secondary to permanent destruction of hair follicles. This is most common with radiation doses over 700 Gy. Most patients are not completely bald following therapy, as hair follicles in the telogen phase at the time of the treatment are able to escape destruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


