(1)
Department of Pathology, Rutgers-New Jersey Medical School, Newark, NJ, USA
Keywords
Cortical stromal hyperplasiaStromal hyperthecosisEndometriomaFollicle cystCorpus luteum cystPolycystic ovaryTheca lutein cystOvarian pregnancySerous cystadenomaSerous tumor of low malignant potentialPapillary serous cystadenocarcinomaMucinous cystadenomaMucinous tumor of low malignant potentialMucinous cystadenocarcinomaPseudomyxoma peritoneiEndometrioid adenocarcinomaClear cell adenocarcinomaBrenner tumorTransitional cell carcinomaCarcinosarcomaBenign cystic teratoma and variantsImmature teratomaDysgerminomaYolk sac tumorOther germ cell tumorsGranulosa cell tumorFibrothecomaSertoli–Leydig cell tumorSteroid cell tumorKrukenberg tumorSex cord tumor with annular tubulesGonadoblastomaGynandroblastoma9.1 Diseases of the Ovaries
The ovary is made up of several tissue types, so it is not surprising that the range of ovarian tumors is so broad. The ovarian surface is lined by the Müllerian epithelium, which is of the same derivation as the mesothelium lining the peritoneal cavity. While older literature theorized an origin of epithelial tumors from this epithelium, current literature suggests different pathogenetic pathways (see below). The germ cells of the ovaries give rise to germ cell tumors. The specialized stroma surrounding the germ cells, the granulosa and theca cells, can give rise to sex cord stromal tumors. In addition, due to blood supply, a large number of neoplasms, both genital and nongenital, can metastasize to ovaries. There are a variety of challenges for pathologic evaluation of ovarian masses (Table 9.1).
Table 9.1
Key points about ovarian pathology
Endometrioma requires evidence of endometrial glandular epithelium and stroma, not just hemorrhage, to confirm the diagnosis histopathologically |
Frozen section diagnosis may be limited in cases requiring extensive sampling for a final diagnosis, particularly large multiloculated mucinous neoplasms |
Hyperplastic ovarian masses are usually bilateral |
Metastatic lesions to ovaries are usually bilateral |
Pseudomyxoma peritoneii is thought to arise from appendiceal mucinous neoplasms, which may coexist with mucinous tumor in the ovary |
9.2 Non-neoplastic Masses
Ovarian masses may be non-neoplastic in nature. Functional cysts are common and often resolve on their own.
9.2.1 Follicle Cyst
The most common functional cyst is a follicle cyst (Fig. 9.1). Follicle cysts are lined by the same cells as normal follicles, with an inner granulosa cell layer, and a visible outer theca interna. The theca externa blends with the ovarian stroma and is not easily detectable on routine stains.
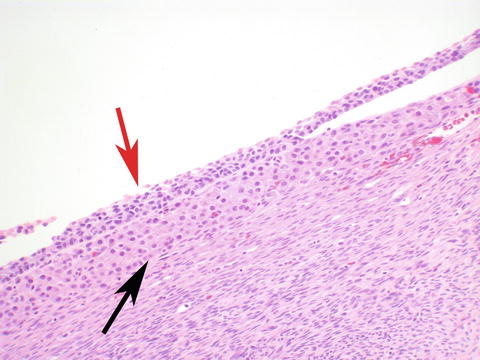

Fig. 9.1
Follicle cyst lined by inner granulosa (red arrow) layer, and outer theca interna (black arrow)
9.2.2 Corpus Luteum Cyst
Corpus luteum cysts (Fig. 9.2) are lined by luteinized granulosa and theca interna cells, in the characteristic cerebriform pattern of folding. Corpus luteal cysts are hemorrhagic in the center. Very rarely, rupture of one of these cysts can cause peritoneal hemorrhage of a degree that may mimic a ruptured tubal ectopic pregnancy.
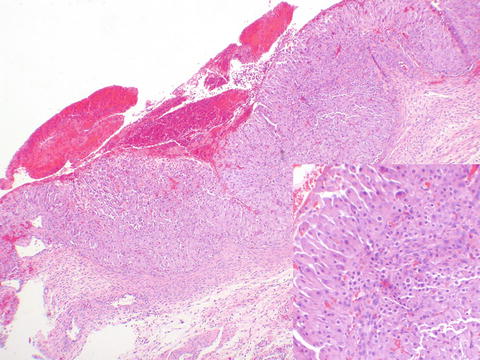

Fig. 9.2
Hemorrhagic corpus luteum showing cerebriform configuration. At higher power, the larger luteinized granulosa, and smaller luteinized theca cells may be seen (inset)
9.2.3 Polycystic Ovary
Polycystic ovaries contain numerous cystic follicles that have not gone on to ovulation, under a dense capsule. Occasionally patients with polycystic ovarian disease do ovulate, so the finding of a corpus luteum or a corpus albicans or two does not rule out the diagnosis, which is a clinical one. Polycystic ovaries may also show cortical stromal hyperplasia and stromal hyperthecosis (see next section).
9.2.4 Cortical Stromal Hyperplasia and Stromal Hyperthecosis
Cortical stromal hyperplasia and stromal hyperthecosis are seen in two clinical instances, polycystic ovarian disease and in menopause. As these findings are hyperplastic, not neoplastic, they are generally bilateral. Cortical stromal hyperplasia is characterized by increased amounts of nodular cortical stroma. Grossly, this may be very yellow in appearance, rarely raising concern for the clinician of a masculinizing sex cord stromal tumor. Histologically cortical stromal hyperplasia appears as dense blue nodular ovarian stroma with its characteristic spindle cells. It is often associated with stromal hyperthecosis, a subtle finding of nests of luteinized stromal cells (Fig. 9.3a, b ), which may be hormonally active, secreting androstenedione. This androgenic compound may be masculinizing, and if the patient is obese, androstenedione may aromatize to estrone in peripheral adipose. This has the potential then of leading to hyperstimulation of the endometrium due to unopposed estrogen.
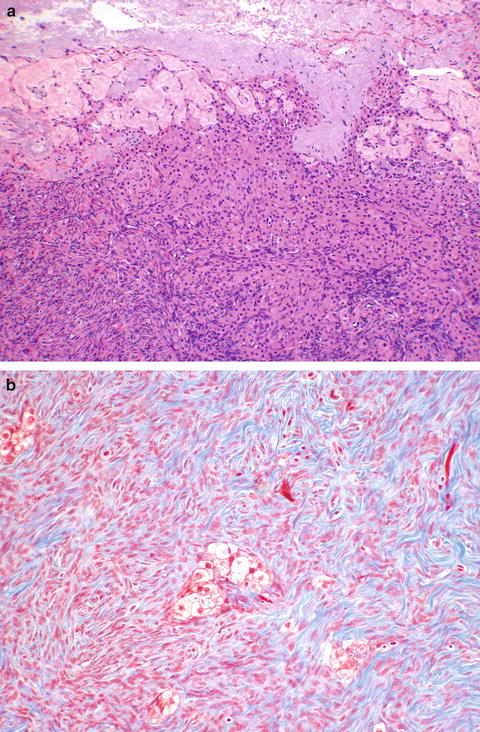

Fig. 9.3
Stromal hyperthecosis. Luteinized stromal cells may be seen adjacent to the pale corpora albicantia (a). Trichrome stain makes these nests more prominent, here seen in the center (b)
9.2.5 Theca Lutein Cysts
Theca lutein cysts are seen in two main clinical scenarios. Ovarian hyperstimulation syndrome after ovulation induction is a clinical diagnosis, and often associated with major fluid imbalance and symptomatology. Although these ovaries are rarely surgical specimens, what is seen are numerous non-ovulated follicle cysts. A similar histopathologic picture is seen with hyperreactio luteinalis, usually presenting in the third trimester, as opposed to the earlier presentation of hyperstimulation syndrome, and seen in association with high levels of beta-hCG, which can occur with molar disease and multiple gestations. Lack of clearance of beta-hCG is thought to be the mechanism of hyperreactio seen with chronic renal disease [1]. Hyperreactio is usually less associated with fluid imbalance, and again, unlikely to provide a specimen for pathology review unless the diagnosis is unsuspected clinically. Both conditions, being hyperplastic rather than neoplastic, are generally bilateral and regress after the hormonal stimulus is removed.
9.2.6 Endometrioma
Endometriosis can form an enlarging ovarian cyst, an endometrioma. The cyst enlarges due to cyclic bleeding. The blood has no egress, so thickens and darkens and becomes “chocolate” fluid. This fluid may compress and destroy the histologically diagnostic lining, so that a diagnosis of endometrioma may only be inferred. To confirm histologically, as in sites elsewhere, endometriosis must show endometrial glandular type epithelium and stroma, not merely evidence of a cyst with old bleeding lined by hemosiderin-laden macrophages.
9.2.7 Ovarian Pregnancy
Not all ectopic pregnancies are tubal, and rarely, an ovarian pregnancy occurs, usually within the corpus luteum (Fig. 9.4). The incidence is quite low, but appears to be greater with assisted reproductive technology [2]. It is possible that a tubal abortion leads to implantation in a rupture site of a corpus luteum. There are specific criteria for the diagnosis of an ovarian pregnancy. Spiegelberg’s criteria, first described in 1878 [3], include that the ipsilateral fallopian tube is intact, the gestational sac must be in the position of the ovary, and this must be attached to the uterus by the uteroovarian ligament, and finally that there must be ovarian tissue in the wall of the gestational sac.
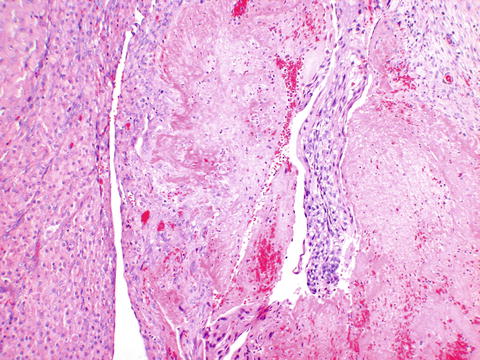

Fig. 9.4
Ovarian pregnancy showing implantation site trophoblasts (right) adjacent to the corpus luteum (left)
9.3 Epithelial Neoplasms
Epithelial ovarian neoplasms were initially thought to derive from the lining of the ovary, the Müllerian epithelium (mesothelium), possibly from invaginations associated with prior ovulation, so-called inclusion cysts. As our understanding of molecular genetic mechanisms has progressed, newer theories have been put forward for epithelial ovarian malignancies, particularly of serous type [4]. This newer evidence suggests that ovarian carcinogenesis falls into two main types, a type I which is low grade and more indolent, and associated with different mutations than the type II aggressive lesions. Papillary serous cystadenocarcinoma, the usual high grade type, is associated with p53 mutations and is now thought to derive from the fimbriated end of the fallopian tube. In tubes removed prophylactically in BRCA individuals, areas of histologic proliferation are sometimes seen. If they show aberrant p53 expression on immunohistochemistry, they are said to show the p53 signature. If, in addition, they show increased proliferation as evidenced by Ki-67 immunostaining, they are designated as serous tubal intraepithelial carcinoma (STIC), thought to be the precursor of high-grade ovarian and peritoneal serous carcinomas. Endometrioid and clear cell carcinomas have been associated with and may derive from endometriosis. Mucinous and Brenner tumor derivations are less clear. A good review of current thinking has been published [4]. The subsequent discussion pertains to the histopathological features of these neoplasms.
9.3.1 Serous Cystadenoma
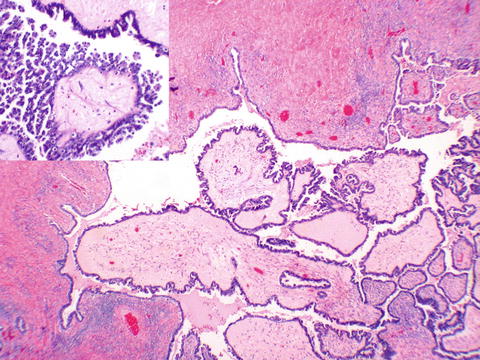
Serous cystadenomas are often unilocular, but may be multilocular. Some are paper thin and can be transilluminated. Grossly, the outer surface is smooth, and the lining is smooth. Histologically, the cyst is lined either by fallopian tubal type epithelium, or the epithelium may have become flattened (Fig. 9.5a–c). A variant neoplasm, papillary serous cystadenofibroma (Fig. 9.5d), has papillary excrescences inside the cystic cavities, but they are lined by benign serous epithelium. It is also possible to have cystadenofibromas lined by other types of Müllerian epithelium.
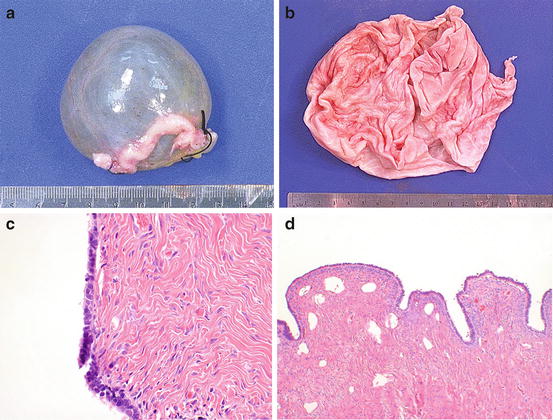

Fig. 9.5
Serous cystadenoma. The lesion is smooth externally (a), and internally (b). The lining (c) resembles fallopian tube epithelium. A variant papillary serous cystadenofibroma shows dense fibrous papillae lined by the same benign serous epithelium (d)
Although not definitively diagnostic, a clue that papillary structures represent a papillary serous cystadenofibroma rather than a low malignant potential tumor is the finding of increased firmness to the touch of cystadenofibroma papillae, due to the stromal predominance of these structures, as well as the finding that they may only occupy a portion of the cyst.
9.3.2 Serous Tumor of Low Malignant Potential (‶Borderline″)
Serous neoplasms of low malignant potential (also called “borderline,” “atypical proliferative serous tumor”) show papillary excrescences inside the cysts that comprise the lesion. These are comprised of fibrovascular cores lined by mildly atypical epithelium, which may show stratification and mitoses. The outer surface of the mass may contain these papillary excrescences as well, or may be smooth. The hallmark of this lesion is the lack of stromal invasion, distinguishing it from an invasive papillary serous carcinoma (Fig. 9.6). Serous tumors of low malignant potential may extend beyond the ovary and are staged the same way as frank malignancies, although they are prognostically better. As such they may have omental implants, which are separated into noninvasive implants (subdivided into epithelial and desmoplastic noninvasive implants), which hug the lobules of omental adipose, and invasive implants, which are thought to be worse prognostically, and invade into the actual adipose. A variant pattern of borderline tumor, the micropapillary pattern, which shows increased generations of thin epithelial fronds, is thought to be the noninvasive variant of low-grade serous carcinoma. If confined to the ovary, the micropapillary variant has similar prognosis to the usual pattern of low malignant potential tumor, but is thought by some authors to be worse than usual borderline tumor when extending beyond the ovary. Nomenclature and prognostication in the area of borderline tumors and tumors in transition between them and frankly invasive carcinoma are still in flux [5].